IAVI has led the search for an HIV vaccine since our founding in 1996
HIV remains one of the deadliest infectious diseases, and developing an HIV vaccine remains a global health priority. Although new treatment and prevention options have changed the HIV landscape for the better since IAVI’s founding, an HIV vaccine is still needed to bring a true end to the HIV pandemic.
At a glance
- 40.8 million people were living with HIV/AIDS in 2024
- 1.3 million people contracted HIV in 2024
- 23% of people living with HIV don’t have access to treatment
- Funding for HIV response was $17B below what is needed to end HIV as a public health threat by 2030, even before the drastic funding cuts of 2025
- 20+ HIV vaccine clinical trials ongoing
Today, our scientists are developing next-generation HIV vaccines to address the complexities of the virus
Many scientific challenges complicate HIV vaccine development:
- HIV on its own is not readily recognized by the immune system. Dense clumps of sugar molecules hide targets on the virus for neutralizing antibodies.
- HIV is enormously genetically variable within and across populations and even with an individual. An effective vaccine will need to block a huge number of genetically variable forms of HIV.
- No one has ever cleared HIV on their own. Because we don’t have a natural model of protective immunity, we don’t have a blueprint for what vaccine-induced immunity should look like.
- HIV stealthily attacks cells of the immune system and inserts its genetic blueprint into them. An effective vaccine will need to stimulate such a strong response that any virus that gets inside the body is immediately neutralized before it starts reproducing inside cells.
Given these complexities, traditional approaches to vaccine development have so far failed to result in a vaccine that provides protection against HIV.
A new approach is needed, and we think we and our partners are on the right path
The origin of our work on a new approach to HIV vaccine design began with the discovery, beginning in 2009, of potent broadly neutralizing antibodies (bnAbs) from large cohorts of individuals living with HIV in Africa, India, Thailand, Australia, the U.K., and the U.S. Researchers at IAVI’s Neutralizing Antibody Center (NAC), a partnership with Scripps Research, and partners isolated and studied these bnAbs, which develop in some people after they have lived with HIV for several years. Later, researchers showed in the lab that some of these bnAbs block HIV from infecting cells.
The discovery of potent bnAbs marked an important milestone in the field and changed our understanding of what might be possible for the development of an HIV vaccine — kicking off a new era of HIV vaccine development.
By investigating how bnAbs engage and inhibit the virus, scientists identified the key parts of HIV that would serve as templates to develop immunogens that could elicit similar bnAbs by vaccination.
In 2021, a study showed that it is possible to protect people from acquiring HIV by giving them infusions of bnAbs. But this worked only when circulating viruses and the bnAbs were very well matched, and the level of bnAbs in the blood were quite high. Still, it was proof of concept that bnAbs can block HIV in people, and it provides support for IAVI’s goal to develop vaccine candidates designed to elicit bnAbs.
IAVI is grateful to the many trial participants and partners who enabled these landmark findings and subsequent vaccine design breakthroughs.
Our understanding of how to design an HIV vaccine evolved with the discovery of Env
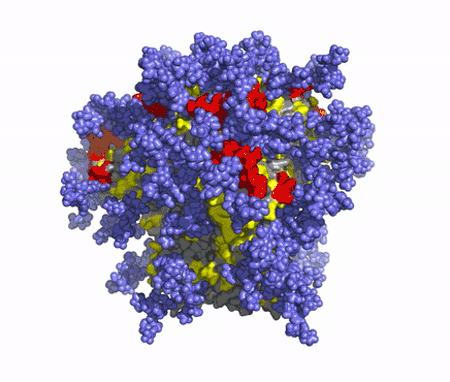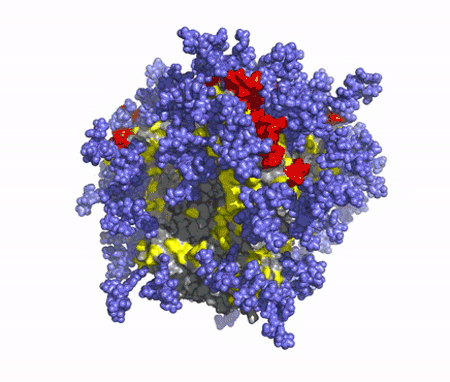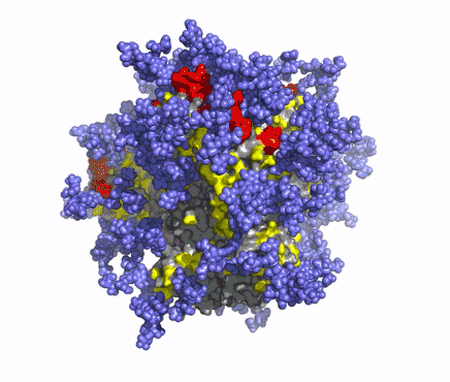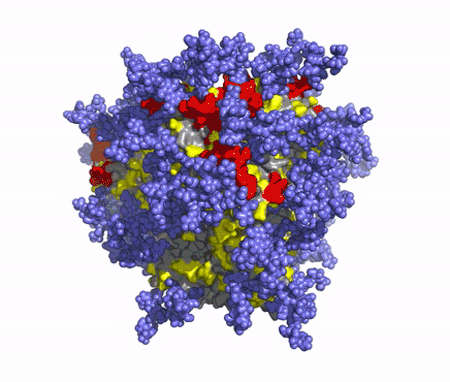
Another major scientific development in understanding how to effectively target HIV was the first high-resolution reports on the structure of HIV envelope (Env), which is the sole target of neutralizing antibodies. For decades scientists were hindered by their inability to capture the precise structure of this notoriously unstable protein. Advances in protein engineering and structural biology platforms enabled high-resolution characterization of the Env protein in unprecedented detail. These findings were detailed in two seminal papers by IAVI NAC investigators and included global research partners in Kenya (KAVI), the Netherlands (AMC), and the U.S. (Cornell, Scripps Research, University of Washington).
Our next-generation vaccine candidates are designed to coach the immune system to produce bnAbs
Given scientific advances over the past decade, many leading scientists think an HIV vaccine must lead to the production of bnAbs to be effective. We think it is possible, through vaccination, to coach the immune system to produce these antibodies.
IAVI scientists at the NAC and their partners at Scripps Research are now attempting to elicit bnAbs via a carefully tailored prime and boosting vaccination approach that targets B cells, the cells of the immune system that produce antibodies. We are using a series of specifically designed HIV vaccine immunogens in a strategy referred to as “germline targeting” to teach the B cells to produce bnAbs.
IAVI’s first trial using the germline targeting approach was successful
The first of these engineered immunogens (eOD-GT8 60mer) developed by IAVI and our partners was shown to successfully engage the target germline B cells in a Phase 1 clinical trial. This result, demonstrating for the first time the ability to activate bnAb precursors by vaccination, marks an important milestone in the field.
In a follow-up Phase 1 clinical trial, IAVI G002, IAVI and partners tested the ability of a second engineered immunogen (Core-g28-v2 60mer) to further guide the responses primed by eOD-GT8 60mer toward the development of bnAbs. In this trial, both immunogens were delivered via Moderna’s messenger RNA (mRNA) technology. A companion study, called IAVI G003, tested the ability of eOD-GT8 60mer, also delivered via Moderna’s mRNA platform, to activate bnAb precursors in African populations. This study, led by African scientists and researchers, was conducted in Africa, and was supported under the ADVANCE program. Results of both studies were published in 2025. The results were encouraging: vaccine receipt in participants initiated the desired immune response with one shot and then drove the response further forward with a different second shot. Another trial in the germline-targeting program will begin in late 2025 or early 2026.
Over the coming years, we’ll continue to develop the germline targeting approach until we reach our goal of a series of vaccines that stimulate production of several classes of bnAbs.
Read more about this new generation of HIV vaccine candidates.
A T-cell vaccine will also be needed
Many scientists think that, in addition to stimulating the development of bnAbs, an effective HIV vaccine will likely need to elicit T-cell responses as well. We’re working on several fronts to advance T-cell based vaccine designs.
Some of the most promising T-cell immunogens in development are specifically designed to address the global diversity of HIV. IAVI is supporting the clinical development of one of these approaches, a so-called conserved HIV immunogen, which combines portions of the virus, or viral epitopes, that are consistent across most of the genetically distinct variants of HIV currently in circulation. A Phase 1 trial was completed in July 2023 and results are forthcoming.
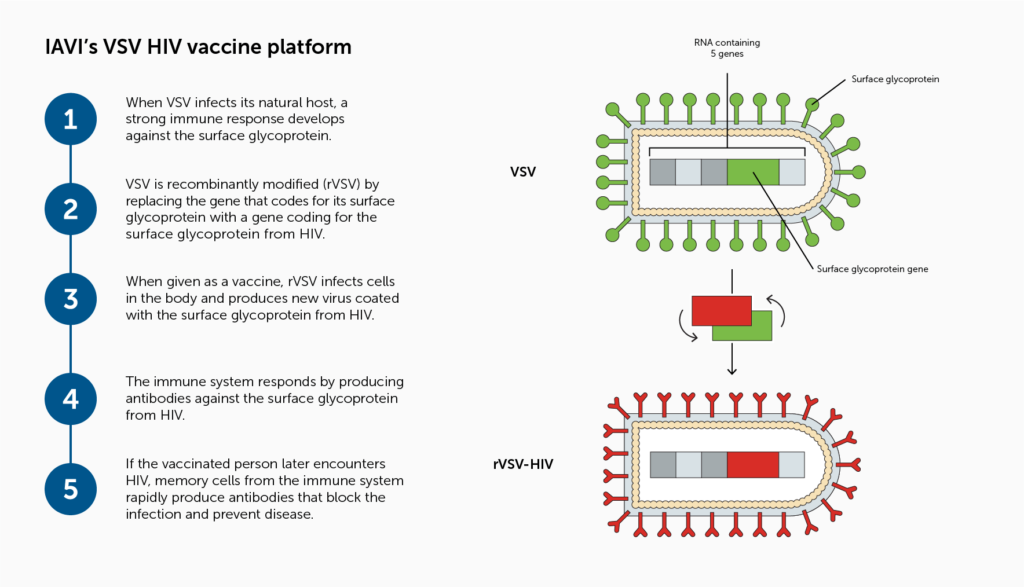
Researchers at the IAVI Vaccine Design and Development Lab are exploring a T-cell vaccine candidate using a recombinant vesicular stomatitis virus (VSV) vector that incorporates an HIV Envelope gene and are testing the vaccine candidate in preclinical studies.