October 8, 2018
IAVI Announces Clinical Trial of Next-Generation HIV Vaccine Candidate Designed to Induce Antibodies to Block HIV Infection
Phase I trial to evaluate safety and immunogenicity of vaccine candidate engineered to elicit targeted immune response against HIV.
NEW YORK — OCTOBER 9, 2018 — The International AIDS Vaccine Initiative (IAVI) announces the start of a Phase I clinical trial (IAVI G001) to test a novel vaccine candidate designed to stimulate the immune system to initiate a key first step in the generation of potent proteins, known as broadly neutralizing antibodies (bNAbs), against HIV. The trial will evaluate the safety of the candidate and the immune responses it is able to induce. The candidate, known as eOD-GT8 60mer, represents an important step forward in the quest to develop an HIV vaccine.
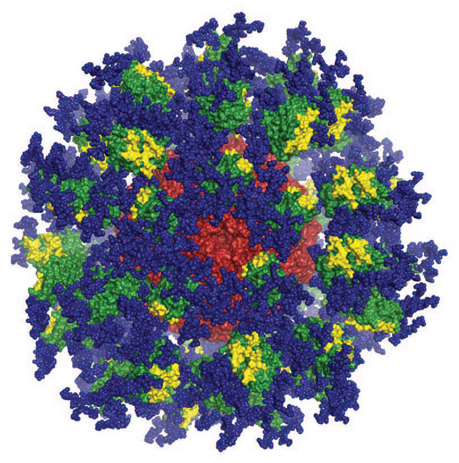 Computer image of the eOD-GT8 immune-stimulating protein. Image courtesy of Joseph Jardine, Sergey Menis, and William Schief of Scripps Research and IAVI.
Computer image of the eOD-GT8 immune-stimulating protein. Image courtesy of Joseph Jardine, Sergey Menis, and William Schief of Scripps Research and IAVI.
Researchers widely agree that a vaccine that induces bNAbs will likely be the best way to confer durable protection against the virus. bNAbs are desirable because in laboratory experiments, they are effective against many of the genetically diverse strains of HIV, and in animal studies, they can block infection of a virus similar to HIV.
“The world urgently needs new ways to prevent HIV infection, and chief among these is a vaccine,” said Mark Feinberg, M.D., Ph.D., president and CEO of IAVI. “Fortunately, a new generation of HIV immunogen candidates, including eOD-GT8 60mer, is entering clinical trials. These candidates are being developed using highly sophisticated and elegant vaccine science and provide a precedent for vaccine strategies targeting the induction of specific immune responses believed to be critical in protecting against HIV infection.”
Over the past 15 years, scientists have gained an unprecedented understanding of the structure of HIV’s outermost envelope protein, which is the target of all bNAbs. From large cohort studies of HIV-infected volunteers, researchers isolated and characterized many bNAbs that develop naturally, but only rarely, during the course of HIV infection, and identified where they bind to the virus. These sites of vulnerability on the virus were then used to design vaccine immunogens, using what is referred to as a structure-based vaccine design approach. The eOD-GT8 60mer candidate is based on one of these sites targeted by bNAbs.
The candidate was developed in the laboratory of Dr. William Schief, director of vaccine design for IAVI’s Neutralizing Antibody Center (NAC) at Scripps Research and professor at Scripps. It is the first candidate to enter clinical trials that was designed using a structure-based vaccine approach, and it is the first in a sequence of engineered vaccine candidates that Dr. Schief and his colleagues are developing.
“In this trial, our goal is to prove that it is possible to induce responses from special, targeted B cells,” said Dr. Schief. “We’ll have a promising outcome if some of the vaccine recipients produce B cells expressing a specific type of antibody, whereas placebo recipients do not. This would confirm that we are able to induce the desired initial immune response, and the next step will be making technical refinements to improve performance.”
There are several steps involved in the development of bNAbs, and in animal studies, the eOD vaccine candidate successfully elicited antibodies that are precursors to the much more specialized antibodies that scientists classify as broadly neutralizing. It is envisioned that eOD or a similar engineered vaccine candidate would be the first in a series of vaccinations that would elicit the bNAbs believed to be needed to protect against HIV infection.
“This is a big moment, not just for HIV vaccines, but for vaccine science as a whole,” said Dr. Dennis Burton, scientific director of the NAC and chair of the Scripps Research Department of Immunology and Microbiology. “This trial is going to tell us how much control we can have over the immune responses induced by a targeted vaccine candidate. If this type of vaccine engineering is successful, it can be applied more broadly, bringing about a new day in vaccinology. If we can really drive immune responses in predictable ways, we can make better, more effective vaccines, not just for HIV but for other viruses, too.”
The IAVI G001 trial will enroll 48 healthy adult volunteers who will receive two doses of eOD-GT8 60mer, along with the AS01B1 adjuvant developed by the pharmaceutical company GSK, or placebo. Adjuvants are substances used to enhance immune responses induced by a vaccine, and the AS01 adjuvant is used in licensed vaccines. The doses are spaced two months apart and are administered through intramuscular injection.
The trial is taking place at two sites: George Washington University (GW) in Washington, D.C., and the Fred Hutchinson Cancer Research Center in Seattle, Washington. At GW, the trial is led by Dr. David Diemert, associate professor in the Department of Medicine, who will serve as the principal investigator at this site, and Dr. Jeffrey Bethony, professor in the Department of Microbiology, Immunology, and Tropical Medicine, who will direct the specimen processing and biorepository aspects of the trial at GW. At the Fred Hutchinson Cancer Research Center, the trial is led by Dr. Julie McElrath, senior vice president and director of the Vaccine and Infectious Disease Division.
The Collaboration for AIDS Vaccine Discovery (CAVD) Comprehensive T-Cell Vaccine Immune Monitoring Consortium/The Dale and Betty Bumpers Vaccine Research Center at the National Institute of Allergy and Infectious Diseases (NIAID)/National Institutes of Health (NIH), the CAVD Comprehensive Antibody Vaccine Immune Monitoring Consortium, and the Fred Hutchinson Cancer Research Center will be performing key analytical assays in support of the trial, to assess whether the targeted immune response is elicited. The CAVD Vaccine Immunology Statistical Center played a major role in the study design and analytical methods to evaluate the data.
Results of the IAVI G001 trial are expected in late 2019.
IAVI developed the eOD-GT8 60mer candidate with support from the Bill & Melinda Gates Foundation, the Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery (CHAVI-ID) at NIAID at the NIH, and Scripps Research.
Research at the NAC that contributed to the development of eOD-GT8 60mer was also made possible by the Dutch people through the Dutch Ministry of Foreign Trade & Development Cooperation and through the generous support of the American people through the United States Agency for International Development (USAID). USAID administers the U.S. foreign assistance program providing economic and humanitarian assistance in more than 120 countries worldwide. The contents are the responsibility of IAVI and do not necessarily reflect the views of USAID or the United States government.
###
About the IAVI Neutralizing Antibody Center at Scripps Research
The IAVI Neutralizing Antibody Center (NAC) was launched by IAVI in 2002 to solve a fundamental problem in HIV vaccine development: the elicitation of antibodies that can neutralize a broad range of HIV variants.Today the NAC is one of the leading sources of innovation worldwide in the study of broadly neutralizing antibodies (bNAbs) and the design of immunogens that could elicit HIV bNAbs in the human body.
During the past decade, through the efforts of the NAC and many other leading scientific collaborators, more than 200 bNAbs have been isolated from volunteers around the world. The structures of some of the most potent of these antibodies and their targets have also been solved. These discoveries are now being applied to the design of novel HIV vaccine candidates.
The NAC is headquartered at Scripps Research in La Jolla, California. Together, Scripps and IAVI employ experts in computational immunogen design, structural biology, virology, immunology, and antibody discovery.
[1]GSK proprietary AS01 adjuvant system contains QS-21 Stimulon® adjuvant licensed from Antigenics LLC, a wholly owned subsidiary of Agenus Inc. (NASDAQ: AGEN), MPL and liposomes.