February 7, 2023
New IAVI-authored paper proposes additional regulatory pathways for filovirus vaccines
Three regulatory experts discuss alternative approaches for demonstrating the effectiveness of vaccines against deadly filoviruses.
In a new perspective piece in Frontiers in Immunology, three regulatory experts discuss alternative approaches for demonstrating the effectiveness of vaccines against deadly filoviruses. Authors include Marion F. Gruber, Ph.D., vice president, public health and regulatory science, IAVI; Steven Rubin, Ph.D., senior director, global regulatory affairs, GSK; and Philip R. Krause, M.D., former deputy director of the Office of Vaccines Research and Review, U.S. Food and Drug Administration. Their lessons learned from Ebola and COVID-19 vaccine development offer possible alternatives to the extraordinary challenge of clinical evaluation during an outbreak.
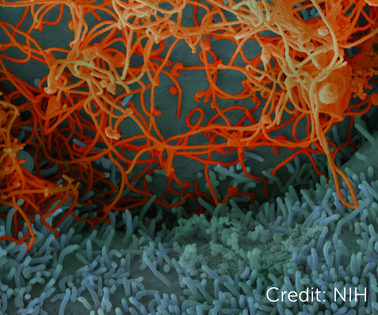 Ebola virus isolated from in November 2014 from patient blood samples obtained in Mali. The virus was isolated on Vero cells. Credit: National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Ebola virus isolated from in November 2014 from patient blood samples obtained in Mali. The virus was isolated on Vero cells. Credit: National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Zaire ebolavirus (ZEBOV), Sudan ebolavirus (SUDV) and Marburg virus (MARV) are all in the filovirus family and can cause severe disease and death in humans and animals. All three cause sporadic outbreaks in sub-Saharan Africa and are considered potential bioterror threats. Vaccines for these filoviruses remain key unmet needs because the immunity provided by ERVEBO®, Merck’s highly efficacious ZEBOV vaccine, is not cross-protective against SUDV and MARV.1
Filovirus outbreaks often ignite and extinguish before clinical evaluation of protective vaccines can take place. Most recently, a ring vaccination trial planned in response to Uganda’s SUDV outbreak did not go forward as planned before the outbreak was declared over.
In such instances, one alternative could be the use of clinical immunobridging studies. These studies would compare filovirus vaccine candidates to licensed filovirus vaccines with demonstrated efficacy as an approach to infer vaccine effectiveness. Clinical immunobridging has the advantage of being able to directly bridge to clinical efficacy data by way of the licensed comparator vaccine. This approach was used for COVID-19 vaccines against COVID-19 variants of concern. Other possible approaches to demonstrate the effectiveness of filovirus vaccine candidates include the use of surrogate endpoints reasonably likely to predict protection, or challenge/protection studies in adequate animal models.
Clinical evaluation remains the gold standard for determining vaccine effectiveness, but proven alternatives could accelerate licensure and eventual delivery of life-saving vaccines during outbreaks. “Regardless of the approach chosen to demonstrate effectiveness of filovirus vaccine candidates,” say the authors, “clinical safety studies to support a favorable benefit risk ratio of the vaccine will be essential. In addition, real world effectiveness studies of the vaccine post-licensure in the event of an outbreak should be conducted to confirm clinical effectiveness.”
Read more about IAVI’s work to accelerate filovirus vaccine R&D and our emerging infectious disease vaccine portfolio.