December 20, 2021
40 years of AIDS vaccine research
A timeline of major milestones in the decades-long effort to develop an HIV vaccine.
There are many success stories over four decades of HIV/AIDS research.
Life-saving antiretroviral therapy, introduced in 1995, is one of the most significant. Writing recently in Nature, Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, recalls that in 1985, a 25-year-old diagnosed with AIDS in the U.S. had a life expectancy of fewer than two years. Whereas, today, a person living with HIV can expect instead to die in old age from causes unrelated to HIV/AIDS.
The heroic efforts of advocates and activists who pressured scientists, governments, and regulators to make these medicines more widely available were another important success story. Advances in HIV prevention science — including using antiretrovirals as pre-exposure prophylaxis — are yet another important success. Since its peak in 2004, AIDS-related deaths have plummeted by 64%.
Together, advances in HIV testing, treatment, and prevention have helped dramatically slow the spread of HIV. But they haven’t stopped it. Last year, 1.5 million people were newly infected with HIV and 700,000 people died from AIDS-related illnesses.
A vaccine is one prevention tool that remains elusive. Research over the past 40 years, as evidenced by this timeline, illustrates just how difficult it is to induce effective immunity against this virus. But with each decade, scientists are making important progress. And, certainly, their persistence is commensurate with that of HIV.
The next decade will likely answer many questions about the feasibility of developing an HIV vaccine and will hopefully bring us closer to the ultimate end of this timeline — the end of AIDS.
Download a PDF of the following timeline.
1980s
As scientists identify and begin grappling with the newly identified retrovirus that causes AIDS, vaccine researchers begin testing protein-based vaccine candidates in the first clinical trials.

1981
U.S. Centers for Disease Control issues a report on June 5 of an unusual spate of pneumocystis carinii pneumonia, among “five gay, otherwise healthy men.” In July, 26 more cases are reported. Those affected are now also developing Kaposi’s sarcoma, which becomes a hallmark of the new disease.
1982
New disease is named acquired immunodeficiency syndrome (AIDS) at a July 27 meeting in Washington, D.C.
1983
Pasteur Institute researchers isolate new retrovirus from the lymphoid tissue of a gay Caucasian patient that may be the cause of AIDS. The virus is later called lymphadenopathy-associated virus (LAV).
First U.S. report of eight infants who had a “disease complex comparable to AIDS.” The children, who were born into families with “recognized risks of AIDS,” primarily intravenous drug use, had unexplained immune deficiencies and some of them had opportunistic infections that fit the description of AIDS.
1984
U.S. scientists confirm discovery of new retrovirus, calling it human T lymphotropic virus (HTLV) type III because they thought it was related to human T-cell leukemia virus (HTLV type I). This discovery prompts U.S. Health and Human Services Secretary Margaret Heckler to proclaim that an AIDS vaccine candidate would be ready for testing within two years.
1985

A new syndrome dubbed Slim disease is reported in Uganda that is strongly associated with HTLV-III.
Anthony Fauci, director of the U.S. National Institute of Allergy and Infectious Diseases (NIAID), quadruples funding for AIDS research. The Division of AIDS is established within NIAID a year later.
1986
International Committee on Taxonomy of Viruses rules that this new virus be called human immunodeficiency virus (HIV).
After attempting therapeutic vaccination in two HIV-infected women from Zaire (now the Democratic Republic of the Congo), French researcher Daniel Zagury inoculates himself and nine HIV-uninfected children in Zaire with a vaccinia viral vector-based candidate, making this the first unofficial preventive AIDS vaccine trial. Zagury is criticized because trial is conducted without regulatory approval or preclinical testing.
1987
A recombinant vaccinia virus vector-based vaccine fails to protect chimpanzees from HIV.
First preventive AIDS vaccine trial begins in U.S. NIAID and the biotech MicroGeneSys test the company’s recombinant gp160 vaccine candidate in 81 HIV-uninfected volunteers, mostly men who have sex with men (MSM).
1988
U.K. Medical Research Council and Uganda Virus Research Institute in Entebbe form Africa’s first research unit focused on determinants of HIV infection.
1990s
Advances in treating HIV/AIDS dramatically alter the landscape of the disease in wealthy countries. Vaccine research begins in earnest with the start of the first Phase III efficacy trial and the creation of multiple initiatives focused on AIDS vaccine development.

1992
Rhesus macaques vaccinated with a live, attenuated simian immunodeficiency virus (SIV), are protected.
1994
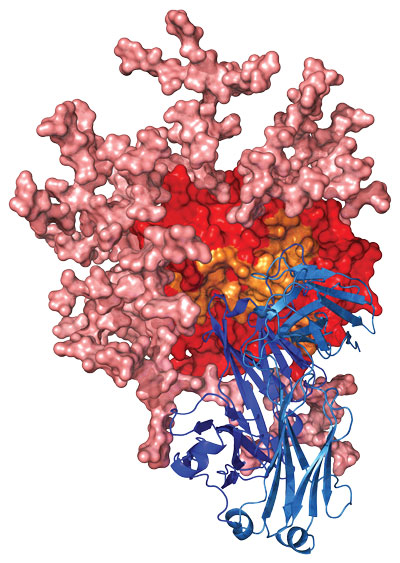
Researchers make a recombinant form of a human antibody called b12 from the bone marrow of an asymptomatic HIV-infected man. It neutralizes more than 75% of HIV strains, making it the first broadly neutralizing antibody (bnAb).
NIAID refuses to fund the first efficacy trial of an AIDS vaccine candidate developed by California-based biotech Genentech. The bivalent vaccine candidate, AIDSVAX B/B, is comprised of recombinant gp120 protein.
1995
Highly active antiretroviral therapy (HAART) is introduced. “From 1985 to 1994 it was all gloom and doom when it came to therapy,” recalls AIDS researcher David Ho, who pioneered the use of protease inhibitors. “Two years later, everything turned around.”
The AIDS Vaccine Advocacy Coalition is formed on December 1, World AIDS Day.
1996
The attenuated strain of SIV previously shown to protect monkeys against SIV infection leads to disease when used to vaccinate infant macaques, dashing hopes for testing this approach in humans.
The International AIDS Vaccine Initiative (IAVI) is created as a non-profit, public-private product development partnership to ensure the development of a safe and effective preventive AIDS vaccine.
1997
At a May 18 speech, U.S. President Bill Clinton announces national goal to develop an AIDS vaccine within a decade. The day is then known as World AIDS Vaccine Day.
1998
Genentech spinoff VaxGen launches Phase III efficacy trial of AIDSVAX B/B, enrolling 5,400 volunteers, mostly MSM, in the U.S., Canada, the Netherlands, and Puerto Rico. A year later, another Phase III trial of AIDSVAX B/E candidate starts in Thailand in a trial of 2,500 injection drug users.
1999
The Vaccine Research Center (VRC) is established on the NIH campus to perform basic research to establish mechanisms of inducing long-lasting, protective immunity against HIV and other pathogens.
Uganda hosts Africa’s first HIV vaccine trial.
The Kenya AIDS Vaccine Initiative (KAVI) and South African AIDS Vaccine Initiative are established.
The HIV Vaccine Trials Network (HVTN) is formed by NIAID.
2000s
Disappointing results from the largest vaccine trial to date testing a candidate that induces T-cell immunity are followed by a surprising result from the RV144 trial — the first to show any evidence that a vaccine can protect against HIV. Meanwhile, key discoveries related to broadly neutralizing antibodies jump-start a new pathway to HIV vaccine development.
2002
NIAID assumes control of the U.S. Department of Defense’s HIV Research and Development Program, which had been preparing to launch a Phase III efficacy trial, RV144, in Thailand testing Sanofi-Pasteur’s canarypox vector-based vaccine candidate ALVAC-HIV (vCP1521) in a prime-boost combination with VaxGen’s AIDSVAX B/E candidate.
2003
Phase III trials show that AIDSVAX is ineffective in either MSMs or IDUs.
RV144 prime-boost trial begins in Thailand in 16,000 mostly low-risk volunteers.
Twenty-four leading AIDS vaccine researchers publish a paper arguing that the insufficient scale of research is impeding development of an AIDS vaccine. Several years later, the Global HIV Vaccine Enterprise is created.
2004
22 prominent researchers publish an article questioning scientific rationale for RV144 trial.
Start of the Phase IIb STEP trial testing whether Merck’s adenovirus serotype 5 (Ad5) candidate can either prevent infection or reduce viral load.
2005
NIAID announces US$300 million over seven years for virtual consortium, the Center for HIV/AIDS Vaccine Immunology (CHAVI).
2006
Researchers in Kenya isolate a subtype A transmitted/founder virus from a six-week-old infant in Nairobi who was HIV infected at birth and enrolled in a mother-to-infant HIV transmission study. This virus sequence is referred to as BG505.
With support from USAID, IAVI initiates large multi-center epidemiology studies. One of them, Protocol G, aims to identify new HIV-specific bnAbs to inform vaccine design efforts.
The Bill & Melinda Gates Foundation awards $287 million to establish the Collaboration for AIDS Vaccine Discovery (CAVD).
2007
Vaccinations in STEP are discontinued because there is no evidence of protection. Subsequent data suggest that vaccine candidate MRKAd5 may have increased risk of HIV among some volunteers. Vaccinations in Phase IIb Phambili trial of same candidate, which launched in South Africa in February, are also halted.
STEP results prompt NIAID to reconfigure Phase IIb PAVE 100 trial of a DNA/Ad5 prime-boost regimen.

2008
A hi-res, 3-D image approximates the trimeric structure of HIV gp120.
Following STEP results, NIAID sponsors summit about shifting funding from clinical development to basic discovery.
The Neutralizing Antibody Center, a partnership of IAVI and The Scripps Research Institute in California, is established.
2009
RV144 results show vaccine candidate reduces risk of HIV infection by about 31%, providing first evidence of vaccine efficacy in humans.
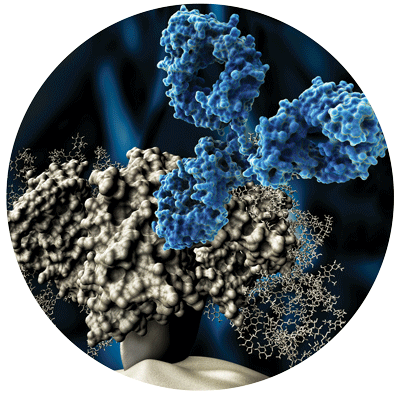
New bnAbs isolated that are more broad and potent, and that target new epitopes on HIV.
The Ragon Institute launches with a $100 million gift, joining researchers from three Boston institutions.
The AIDS Vaccine Conference draws a record number of attendees as the field seems more optimistic about the prospects for HIV vaccine development.
2010s
Researchers are exploring multiple paths to HIV vaccine development. One path is to design and test mosaic vaccine antigens that are meant to address HIV’s overwhelming global diversity. Another is to explore the role of non-neutralizing antibodies in the role of HIV protection. At the same time, researchers hone in on HIV’s elusive structure and use this information to design vaccine candidates to induce broadly neutralizing antibodies.
2010
Mosaic antigens, computationally derived to provide maximum coverage against HIV, show promise in monkeys.
Researchers analyze the structure of recently identified bnAbs to determine how they bind to and neutralize HIV. This work shows the extensive affinity maturation that gives rise to these antibodies, which has consequences for vaccine design.
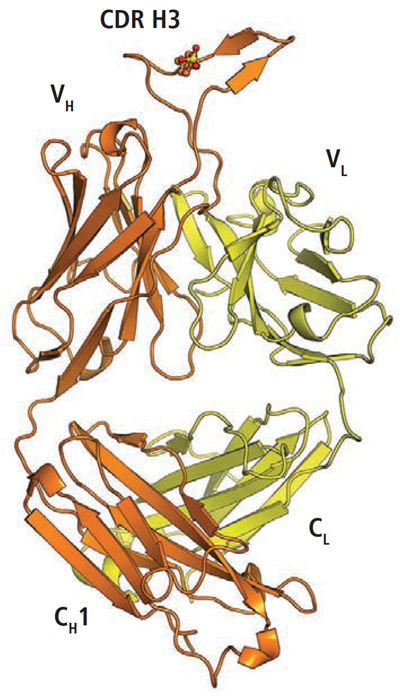
Researchers show that cell-to-cell spread of HIV in vitro is about 10 times more efficient than infection spread by free-virus particles.
In the wake of the RV144 results, researchers home in on the role non-neutralizing antibodies may play in protection against HIV.
Researchers identify crystal structure of PG16, one of the recently identified bnAbs, revealing its unique features.
The Pox-Protein Public-Private Partnership (P5) collaboration is established to explore and expand on the results of the RV144 trial — the only vaccine candidate to show any efficacy in preventing HIV infection.
IAVI Report profiles Bette Korber of the Los Alamos National Laboratory on HIV evolution and diversity.
2011
Researchers continue isolating dozens of new broadly neutralizing antibodies and gather clues about how they develop in infected individuals.
Vaccination with a replication competent CMV vector-based vaccine leads to viral control in SIV-challenged monkeys.
HVTN 505 vaccine trial is expanded to explore whether the DNA/Ad5 prime-boost regimen can block HIV infection.
Researchers from Emory and Beth Israel Deaconess Medical Center are selected to lead a 5-year, $60 million effort to use NHP models to understand mucosal HIV transmission.
Researchers debate how adaptive clinical trial designs could speed the evaluation of vaccine candidates.
IAVI Report interviews four leading researchers on what it will take to go from broadly neutralizing antibodies to vaccine immunogens.
2012
Additional analyses of RV144 results provide some insight into what types of immune responses may have contributed to protection.
The U.S. NIAID announces two consortia known as the Center for HIV/AIDS Vaccine Immunology & Immunogen Discovery (CHAVI-ID) to tackle scientific obstacles to HIV vaccine development. The two centers were established at Duke University and The Scripps Research Institute (TSRI) in La Jolla, CA.
IAVI-Translational Health Science and Technology Institute (THSTI) HIV Vaccine Translational Research Laboratory opens in India to study genetic and virological properties of circulating HIV strains in India.
2013
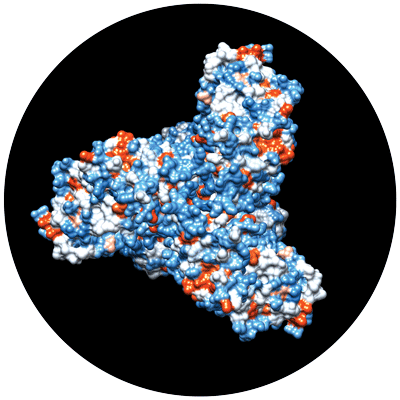
Scientists at TSRI and IAVI apply computational and genetic engineering techniques to generate a virus-like particle (eOD-GT6) that activates bnAb precursors in the lab — an initial step in developing a vaccine immunogen capable of setting off the development of bnAbs.
Using BG505 SOSIP.664, researchers capture cryoelectron microscopy and x-ray crystallography images of a native-like HIV Env trimer.
The Bill & Melinda Gates Foundation awards a grant to IAVI to create the Vaccine Product Development Center (VxPDC) to assist investigators affiliated with the Foundation’s Collaboration for AIDS Vaccine Discovery with the complex process of transitioning vaccine candidates from the laboratory to the clinic.
IAVI expands the Product Development Center (PDC) to support other products in addition to vaccines and extends its translational expertise to other partners.
The Human Vaccines Project launches to accelerate the development of new vaccines by decoding the human immune system.
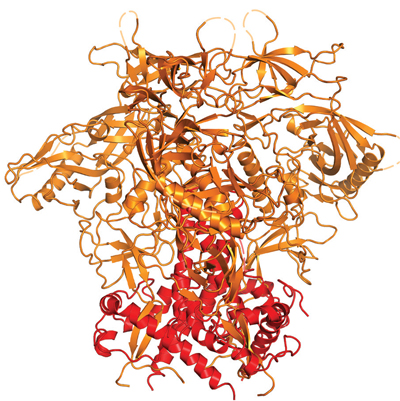
Researchers publish data on a stabilized HIV trimer protein known as BG505 SOSIP.664 based on an isolate from an HIV Clade A-infected infected infant in Kenya.
Immunizations in the Phase IIb HVTN 505 trial are stopped prematurely because the DNA/Ad5 prime-boost vaccine regimen was not effective at either preventing HIV infection or lowering viral load in individuals who acquired HIV despite vaccination.
Researchers at the VRC announce plans to begin a passive immunization clinical trial with the VRC01 bnAb.
Two studies published in Science describe the structure of the notoriously unstable HIV trimer, a long-term goal of HIV vaccine researchers. This helps accelerate structure-based vaccine design efforts.
2014
Researchers report on the identification of bnAbs from a South African donor, CAP256, which have increased breadth and potency.

The bnAb PIGDM1400 is identified and is among the most broad and potent antibodies isolated to date.
With support from USAID through PEPFAR, IAVI launches Vaccine Immunology Science and Technology for Africa (VISTA) and the International Training Program to strengthen research capacity for HIV vaccine research in sub-Saharan Africa.
2015
Researchers make progress in developing native-like HIV Env trimers as vaccine immunogens.
2016
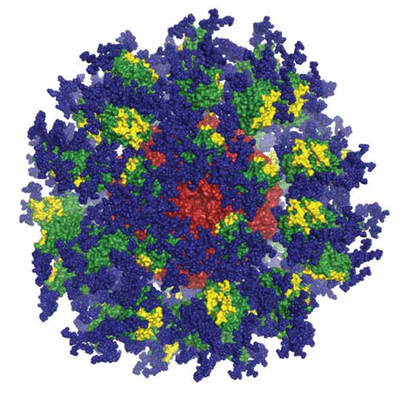
Studies in transgenic mice show that the engineered eOD-GT8 60mer immunogen followed by a native like timer immunogen can induce VRC-01-like antibodies.
The AMP studies are launched to test whether intravenous infusions of the bnAb VRC01 is effective at preventing HIV infection.
The Phase IIb/III HVTN 702 trial, known as Uhambo, is launched to test whether a modified version of the vaccine candidate tested in RV144 is effective at preventing HIV infection in South Africa.
2017
Researchers generate a trispecific HIV bnAb that can protect against a modified simian/human immunodeficiency virus in macaques.
Researchers at the VRC isolate the N6 bnAb that can neutralize 98% of HIV isolates.
The Imbokodo or HVTN 705/HPX2008 Phase IIb trial begins in South Africa. It is designed to test the efficacy of an Ad26-vector-based HIV vaccine candidate that delivers mosaic antigens designed to induce immune responses against a wide variety of global HIV strains.
2018
The European AIDS Initiative 2020 program works to advance several native-like HIV Env trimer immunogens into clinical trials.
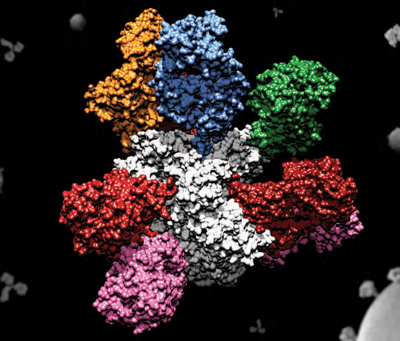
IAVI begins the first clinical trial of the eOD-GT8 60mer vaccine immunogen, which is designed to stimulate the immune system to initiate a key first step in the generation of bnAbs.
Several trials testing passive administration of HIV broadly neutralizing antibodies are underway by the VRC and researchers at Rockefeller University.
IAVI expands its focus to include developing vaccines against emerging infectious diseases, including Lassa fever and TB.
IAVI and the Serum Institute of India partner to develop and manufacture globally affordable and accessible antibody products for HIV.
2019
The European Union approves an Ebola vaccine that utilizes a vesicular stomatitis virus vector, which is also being explored for HIV vaccines.
The Mosaico or HVTN 706/HPX3002 Phase III trial begins in North and South America and Europe testing the efficacy of an Ad26-based mosaic HIV vaccine candidate in men who have sex with men and transgender people.
IAVI starts a Phase I clinical trial (IAVI W001) of a native-like HIV envelope vaccine candidate (BG505 SOSIP.664 gp140) in Nairobi, Seattle, and Boston.
NIAID awards the third seven-year grant to the Duke Consortia for HIV/AIDS Vaccine Development and the second seven-year grant to the Scripps Consortium for HIV/AIDS Vaccine Development to develop and test vaccine candidates.
IAVI Report publishes a special issue on African-led HIV vaccine science.
2020s
More disappointing results from clinical trials are reported, causing many in the field to coalesce around the idea that an effective HIV vaccine will need to induce broadly neutralizing antibodies against the virus. In pursuit of that goal, researchers push ahead with structure-based immunogen design efforts.
2020
Vaccinations in the Phase IIb/III HVTN 702 or Uhambo trial are stopped early because the vaccine candidate did not provide protection.
The PrEPVacc trial begins enrollment. This African-led Phase IIb/III clinical trial is evaluating two experimental HIV vaccine regimens compared to placebo, as well as a new PrEP combination pill known as Descovy. This is the first time that oral PrEP and experimental vaccines are being tested at the same time.
IAVI, Scripps Research, and the NIH agree to pool their HIV bnAb assets and expertise to develop a combination bnAb product specifically designed to be available and affordable globally.
Decades of research on HIV facilitates the rapid development of vaccines against SARS-CoV-2, a novel coronavirus that spreads globally and kills millions.
2021
Following the results of the Uhambo trial, more and more researchers in the field converge on the idea that an effective vaccine will need to induce so-called bnAbs.
Results of the Antibody Mediated Prevention trials show that the antibody VRC01did not protect against HIV infection any better than placebo.
Vaccinations begin in Zambia for a Phase I trial testing a mosaic HIV vaccine candidate known as HIVconsvX.

IAVI and Scripps Research announce that an experimental HIV vaccine candidate (eOD-GT8 60mer) primed the immune system as the first stage in the production of bnAbs in a Phase I clinical trial (IAVI G001).
Data from the Phase IIb Imbokodo trial show that the mosaic-based HIV vaccine regimen did not provide sufficient protection against HIV infection.
IAVI Report surveys leading researchers, funders, and policymakers on what the AMP results mean for the field.
IAVI commemorates 25 years of translating scientific discoveries into affordable, globally accessible public health solutions. Learn the history.
Photo/image credits: Andreas von Bubnoff; Vaccine Research Center at NIAID; Sriram Subramaniam, NIH; Vanessa Vick; Jean-Marc Giboux/Getty Images; Robert Pejchal, The Scripps Research Institute; PLoS Pathog. 9(9): e1003618, 2013; Peter D. Kwong, Marie Pancera, and Jonathan Stuckey, NIH/NIAID/VRC; Joseph Jardine, Sergey Menis, and William Schief, Scripps Research and IAVI. Alba Torrents de la Peña, Amsterdam University Medical Center; Viruses, April 1: 10(4), 2018; NIAID; Hailee R. Perrett, Scripps Research