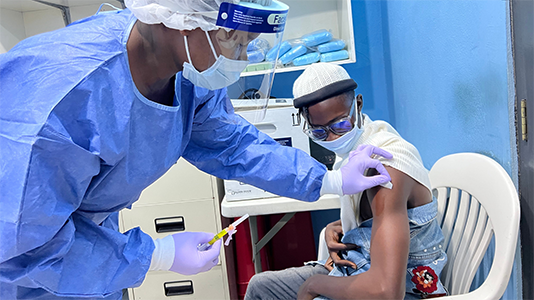
A platform approach to vaccine development
All of IAVI’s emerging infectious disease vaccines use the same technology behind Merck’s Ebolavirus vaccine, ERVEBO®, which is licensed in more than a dozen countries.
Lassa virus (LASV) vaccines: Bridging the gap for an endemic threat
IAVI and our partners are developing a single-dose vaccine candidate for protection against LASV. No vaccine is yet approved to prevent Lassa fever.
LASV (family Arenaviridae) causes an acute viral hemorrhagic illness called Lassa fever. An estimated 5,000 people die each year from Lassa fever, and about 300,000 people fall ill across West Africa annually – though the true disease burden is thought to be much higher. Given its potential to cause a public health emergency of international concern, LASV is included in the World Health Organization’s Pathogens prioritization: a scientific framework for epidemic and pandemic research, which identifies pathogens for which there is an urgent need for accelerated R&D and countermeasures.
IAVI’s LASV vaccine candidate (rVSVΔG-LASV-GPC) is currently in Phase 2a clinical development (IAVI C105, NCT05868733). It is the most advanced LASV candidate to date. In this study, researchers are evaluating the vaccine candidate’s safety, tolerability, and immunogenicity at two different dosage levels in adults, including people living with HIV, as well as in adolescents and in children two years of age and older. The vaccine candidate was well-tolerated and immunogenic among participants in IAVI’s Phase 1 LASV vaccine trial (IAVI C102, NCT04794218), which was conducted in the U.S. and in Liberia. Robust immune responses appear to be sustained for up to one year after vaccination.
IAVI’s Phase 2a LASV vaccine clinical trial is funded by the Coalitions for Epidemic Preparedness Innovations (CEPI) and the European and Developing Countries Clinical Trials Partnership. Earlier work was funded by CEPI.
More about LASV
- No vaccines or specific treatments available
- Transmitted via animal reservoir (rats) and direct contact with a symptomatic person or their infectious bodily fluids
- Causes a range of symptoms including flu-like symptoms; 20% of people with LASV infection develop serious symptoms including widespread bleeding and major organ failure
- Some survivors experience permanent deafness


Regional commitment
In 2025, West African Ministers of Health pledged their joint commitment to advance the development of, and readiness for, much-needed vaccines against Lassa fever in recognition of the significant threat to regional health security posed by the disease.
The pledge includes supporting the development of rVSVΔG-LASV-GPC through a collaborative co-funding approach and joint action to mobilize and secure resources through advocacy and regional coordination.
A game-changing approach
In West Africa, seasonal outbreaks of Lassa fever place a significant burden on communities and regional health systems. There are currently no licensed vaccines to combat Lassa in endemic countries and to stop potential global spread.
IAVI’s vaccine candidate is currently the most advanced of any Lassa vaccine in development. We sat down with Gaudensia Mutua, a medical director at IAVI, to discuss the promising Phase 1 results that support our ongoing Phase 2a clinical trial. Watch this IAVI Impact video to learn about how IAVI’s Lassa vaccine R&D model is changing the game.

LASV by the numbers
0
LASV vaccines available
80%
Combined mortality rate for both mother and child during infection in third trimester of pregnancy (source)
News & Articles

Frequently asked questions: Lassa fever
September 11, 2025
West African leaders commit to advance Lassa fever vaccine for the region
September 09, 2025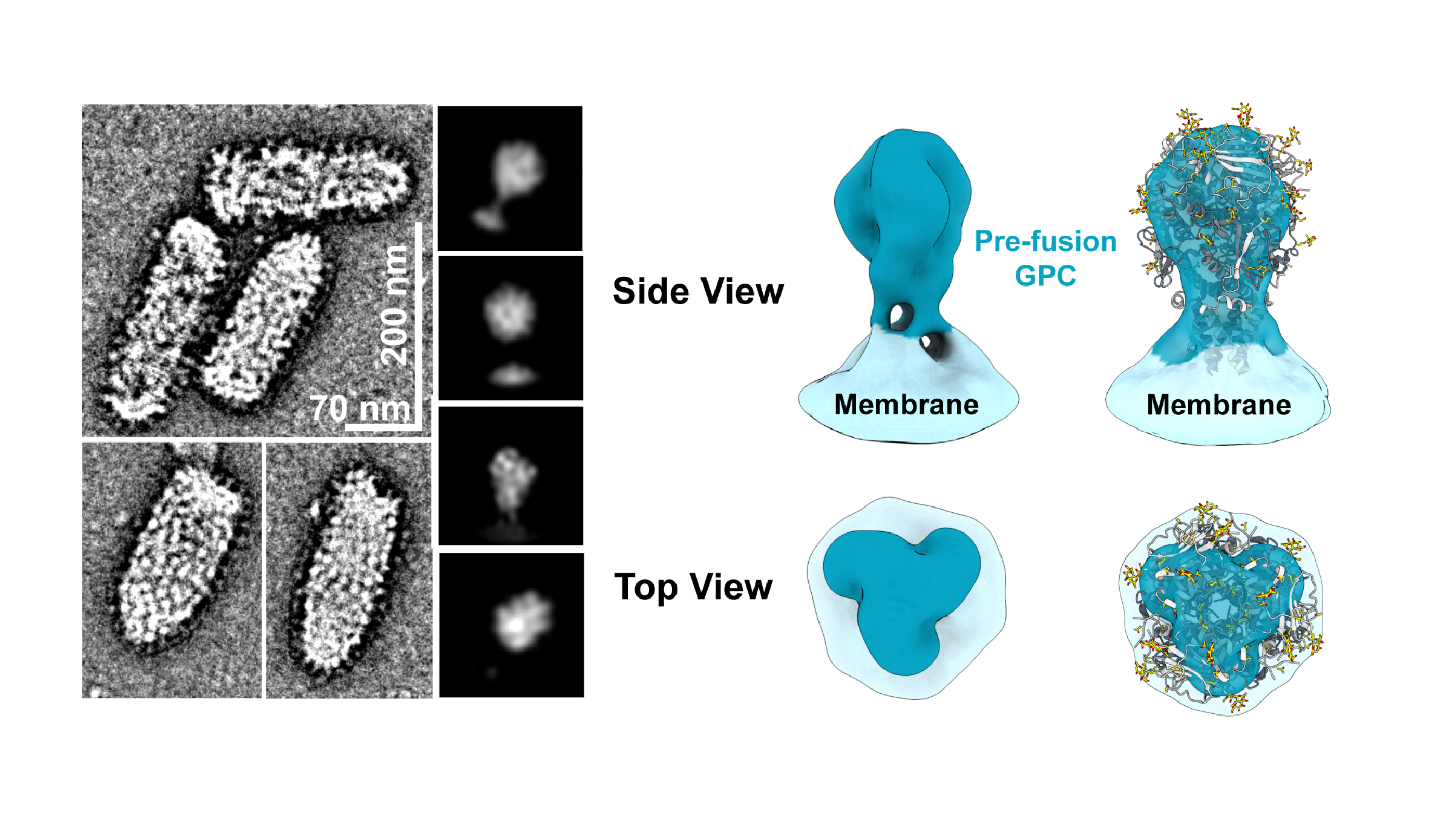
Findings from papers in eBioMedicine advance development of IAVI’s single-dose Lassa fever vaccine candidate
April 16, 2025
Participants in Nigeria vaccinated in first-ever Phase 2 Lassa fever vaccine clinical trial, sponsored by IAVI
April 04, 2024