January 13, 2021
Researchers demonstrate protection offered by novel TB vaccine candidate in animal model
- A study of MTBVAC efficacy in a macaque model of tuberculosis shows that the vaccine candidate protects against aerosol infection with Mycobacterium tuberculosis.
- Researchers from Europe and the U.S. demonstrate that the MTBVAC vaccine protects better than the current BCG vaccine in a model of tuberculosis in macaques and compare the immunological patterns to those conferred by MTBVAC in clinical trials.
JANUARY 13, 2021 – The tuberculosis (TB) vaccine candidate MTBVAC takes a new step as a potential candidate for universal vaccination against TB and an alternative to the current vaccine (BCG), based on results of research published in the journal NPJ Vaccines. The study published January 4, 2021, demonstrates the significant protection offered by MTBVAC compared to BCG in a model of respiratory tuberculosis in rhesus macaques.
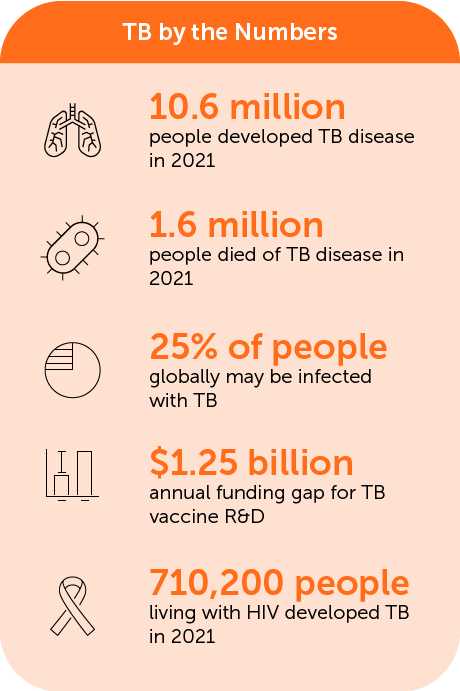
Despite World Health Organization (WHO) declaration of TB as global health emergency in 1993, today the disease continues to be one of the leading causes of mortality caused by infectious diseases worldwide. The WHO Global Tuberculosis Report 2020 estimates that 1.4 million people died of TB in 2019, and it is a leading cause of death of people living with HIV. It is estimated that, as a consequence of the COVID-19 pandemic, deaths from TB could increase by up to 20% in the next five years.
The current BCG vaccine, based on a live attenuated form of Mycobacterium bovis isolated from cows and which in 2021, marks 100 years since its first use in humans as a TB vaccine, continues to be the only licensed vaccine against the disease. After decades of research in this field, MTBVAC is the first and only vaccine candidate based on the human pathogen Mycobacterium tuberculosis to have entered human clinical evaluation, a historic milestone in human vaccinology. MTBVAC has demonstrated a favorable safety profile and was well-tolerated and immunogenic in Phase I studies of adults in Switzerland and newborns in South Africa. Two Phase II studies are ongoing in South Africa in healthy newborns and in TB- infected and uninfected adults. The results of these studies will provide an expanded safety and immunogenicity profile and allow for dose selection with a larger number of participants to support advanced Phase III efficacy evaluation in the target age groups.
The article by White et al., led by Dr. Sally Sharpe (Public Health England), shows that a single dose of MTBVAC administered intradermally confers significantly better protection against aerosol exposure to M. tuberculosis in rhesus macaques compared to intradermal administration of BCG at the same dose. Vaccination with MTBVAC resulted in significant reduction in disease pathology induced by M. tuberculosis infection as measured by medical scan imaging in vivo, macroscopic pathological lesions examination, and pathological anatomy study of the frequency and severity of pulmonary granulomas.
The publication shows for the first time the immune responses induced after vaccination with MTBVAC in macaques and compares them with the immunological patterns studied in the previous Phase I trials in adults (Spertini et al. Lancet Respiratory Medicine 2015) and in infants (Tameris et al. Lancet Respiratory Medicine 2019), as well as in the ongoing Phase IIA trials in adults (NCT02933281) and in infants (NCT03536117). The results by White et al. show that the immunological profiles induced after vaccination with MTBVAC and BCG reflect those identified in human clinical trials of MTBVAC.
The production of specific T-cell cytokines following stimulation with MTBVAC and M. tuberculosis peptide combination revealed a predominantly Th1 response of multifunctional CD4 T cells (IFN-γ + TNF-α + IL2 +). T-cell populations were detected in which the secretion of specific IFN-γ increased following stimulation with CFP10 protein (present in MTBVAC and absent in the current BCG vaccine), confirming previous studies in mouse models that showed a correlation of protection with CFP10-specific response (Aguilo et al Nature Communications 2017). These results, added to those recently published by the team at the University of Zaragoza in a mouse model (Pérez et al EBioMedicine 2020), indicate that MTBVAC constructed from a clinical strain belonging to lineage 4, the most frequently transmitted lineage in Europe, Africa, and America, also protects against the M. tuberculosis lineages 2, 3, and 4, which are the most widespread strains throughout the world, including Asia, allowing anticipation of good protection of MTBVAC against the main strains of M. tuberculosis circulating globally. Recent publications of the group indicate that MTBVAC confers a nonspecific immunity in human monocytes similar to that conferred by BCG, also called “trained immunity,” as well as protection against pneumococcal pneumonia in mouse model (Tarancon et al. PLoS Pathogens).
The study published by NPJ Vaccines consolidates previous preclinical and clinical safety and immunogenicity studies and represents a strong proof of concept of the efficacy of MTBVAC in the macaque model, the most relevant model of efficacy against respiratory TB. These findings support the clinical development of studies to demonstrate the efficacy of MTBVAC as a prophylactic vaccine against respiratory TB in humans, which could potentially make MTBVAC an essential tool in the fight against this disease.
About MTBVAC
MTBVAC is the only vaccine candidate against TB in clinical trials based on a genetically modified form of the pathogen isolated from humans Mycobacterium tuberculosis which, unlike BCG, contains all the antigens present in strains that infect humans. This vaccine candidate was constructed in the laboratory of the University of Zaragoza, which has formed part of CIBERES from the beginning of the project, in collaboration with Dr. Brigitte Gicquel of the Pasteur Institute in Paris. Currently, the University of Zaragoza has as an industrial partner the Spanish biotechnology company BIOFABRI, responsible for the industrial and clinical development of MTBVAC, studying its immunity and safety in two Phase IIa trials in babies and adults in South Africa. Vaccine efficacy studies are expected to begin next year. For the clinical development of MTBVAC, the TB vaccine project has received the advice and support of the European TBVI since 2008 and since 2016 with IAVI for clinical development in adults.
About Biofabri (Zendal group)
BIOFABRI is a Spanish biopharmaceutical company created in 2008 with the vision of researching, developing and manufacturing vaccines for humans. BIOFABRI focuses on human health, with strong technical, development, and manufacturing capabilities and a proven track record. Biofabri belongs to Zendal, a Spanish biopharmaceutical business group that specializes in the development, manufacture and commercialization of biotechnology and pharmacy products, both for humans and animals. Biofabri is also the manufacturer of MTBVAC. www.biofabri.es
About the University of Zaragoza
The University of Zaragoza is the main center for technological innovation in the Ebro valley and has great prestige among Spanish and European universities. The University of Zaragoza participates in various exchange programs, collaborating with universities and research centres in Europe, Latin America, and the U.S., thus strengthening its international position. Microbiologists from our university belonging to CIBERES lead the research and discovery of MTBVAC. Within the TBVI consortium, the MTBVAC discovery phase has included rigorous preclinical characterization by independent laboratories and research groups. Biofabri is the industrial partner of the University of Zaragoza, responsible for the industrial and clinical development of MTBVAC.
About CIBERES
The Biomedical Research Network Center (CIBER) for Respiratory Diseases (CIBERES) is a consortium dependent on the Carlos III Health Institute (Ministry of Science and Innovation) and co-financed with FEDER funds. The purpose of the CIBER for Respiratory Diseases (CIBERES) is to promote and facilitate the investigation of respiratory diseases through research of excellence and its rapid and safe transfer to clinical practice. Created in 2007, CIBERES currently brings together about 400 researchers from nine autonomous communities who work together in three scientific programs, which integrate the following lines of research: lung cancer, sleep apnea, pulmonary fibrosis, pulmonary hypertension, asthma, injury acute pulmonary disease, tuberculosis, pneumonia, chronic obstructive pulmonary Disease (COPD), and new therapeutic targets.
About TBVI
The Tuberculosis Vaccine Initiative (TBVI) is a nonprofit foundation that facilitates the discovery and development of new tuberculosis vaccines that are safe and effective, accessible, and affordable for all people. TBVI integrates, translates, and prioritizes research and development efforts to discover and develop new tuberculosis vaccines and biomarkers for global use. TBVI provides essential services that support the R&D efforts of its 50-partner consortia from academia, research institutes and private industry in the TB vaccine field. These services include the identification, design and development of projects; projects management; resource mobilization; knowledge development, exchange and networking. Technical advice and support for clinical and product development. www.tbvi.eu
About IAVI’s TB Vaccine Clinical Research
IAVI is currently developing two new TB vaccine candidates in clinical trials.
- A Phase Ib / IIa (A-050) dose definition safety and immunogenicity study of the live attenuated vaccine candidate MTBVAC in South Africa. MTBVAC is being compared with BCG in adults with and without latent tuberculosis infection.
- A Phase II study (A-055) involving adults from South Africa and Tanzania. The Statens Serum Institut’s H56 TB protein vaccine candidate, in combination with the adjuvant IC31®, developed by Valneva, is being evaluated in a trial sponsored by the Statens Serum Institut and IAVI South Africa NPC (formerly known as Aeras Global TB Vaccine Foundation NPC) to see if it reduces the rate of recurrent tuberculosis in HIV-uninfected adults who have already been successfully treated for pulmonary tuberculosis disease.
Organizations Involved in the study
- Public Health England, National Infection Service, Porton Down, Salisbury SP4 0JG, UK
- The Churchill Hospital, Headington, Oxford, UK
- La Jolla Institute for Allergy and Immunology, La Jolla, CA, U.S.
- Biofabri, Ponteverdra, Spain
- Mycobacteria Genetics Group (Grupo de Genética de Micobacterias) Departament of Microbiology, University of Zaragoza, IIS-Aragón, CIBERES, Zaragoza, Spain.
- IAVI