May 10, 2023
New IAVI paper makes the case for strengthening clinical and regulatory pathways to accelerate global access to monoclonal antibodies
Expanded access would benefit many in low- and middle-income countries.
In a new, peer-reviewed Current Opinion piece in Pharmaceutical Medicine, IAVI’s global access experts make the case for streamlining and strengthening clinical and regulatory pathways for monoclonal antibodies. Authors include Shelly Malhotra, executive director, global access, IAVI; Ayesha Sitlani, associate vice president, novel technologies and platforms; Vincent Muturi-Kioi, senior medical director, IAVI; and Lisa Gieber, senior information specialist, IAVI.
Monoclonal antibodies (mAbs), based on the body’s natural antibodies, are a trusted prevention and treatment product for many infectious and non-communicable diseases. The first monoclonal antibody product was licensed 30 years ago for the prevention of liver transplant rejection. In the years since, mAbs have been developed to prevent and treat many illnesses with high morbidity and mortality, including COVID-19 and sickle cell anemia.
Despite bearing a high mortality and morbidity burden of the illnesses that can be addressed with mAbs, low- and middle-income countries (LMICs) are not reaping the health and economic benefits that would come from their widespread use. Fewer mAbs are licensed for use in LMICs; even when regulatory approval is granted, pricing structures frequently mean their use is out of reach in resource-challenged environments. The experiences of many LMICs during the COVID-19 pandemic shed light on the current challenges for clinical development and regulatory approval: there are six SARS-CoV-2-targeting mAb products licensed as of late 2022 in high-income countries, but only a handful licensed in LMICs. In sub-Saharan Africa, only one mAb has been licensed.
The clinical development and regulatory approval processes in many LMICs could be strengthened. There are many complex and sometimes undefined or absent local regulations for the development and testing of new mAbs, which may be further confounded by multiple layers of approvals for clinical trials.
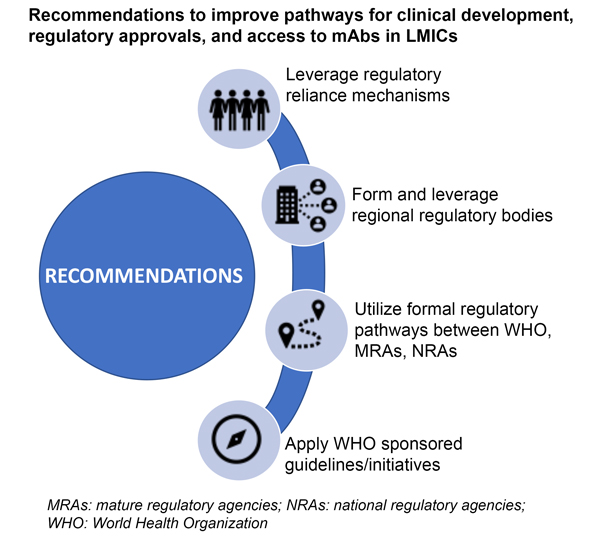
The authors of the paper make several recommendations to speed up global access to mAbs, as described in the graphic below. Countries should work to adopt centralized and collaborative regulatory processes across regions, which can minimize redundancies and accelerate access to these lifesaving products. The World Health Organization (WHO) has put in place several global access initiatives designed to speed the use of mAbs through developing guidelines for preferred products and prequalification. To best take advantage of these opportunities, product developers should engage early in the process with regulatory agencies and the WHO.
Read more about IAVI’s work to accelerate access to vaccines and therapeutics in low- and middle-income countries.