September 20, 2022
IAVI scientists publish findings that suggest intranasal vaccination can prevent COVID-19 breakthrough infections in small animal models
Preclinical results support intranasal delivery of IAVI’s COVID-19 vaccine candidate.
Scientists at IAVI’s Vaccine Design and Development Laboratory (DDL) in New York working in collaboration with scientists at Merck & Company, Inc., recently published research demonstrating that a single dose of IAVI’s COVID-19 vaccine candidate delivered to the oral mucosa and upper respiratory tract of hamsters induced immunity that protected them from SARS-CoV-2 infection. DDL and Merck scientists developed the candidate using the same recombinant vesicular stomatitis virus (rVSV) vaccine delivery technology used for Merck’s now-licensed Ebola Zaire vaccine ERVEBO®. This rVSV technology also is the basis of the candidates in IAVI’s emerging infectious diseases (EIDs) vaccine development portfolio. Read more in IAVI’s EID vaccines fact sheet.
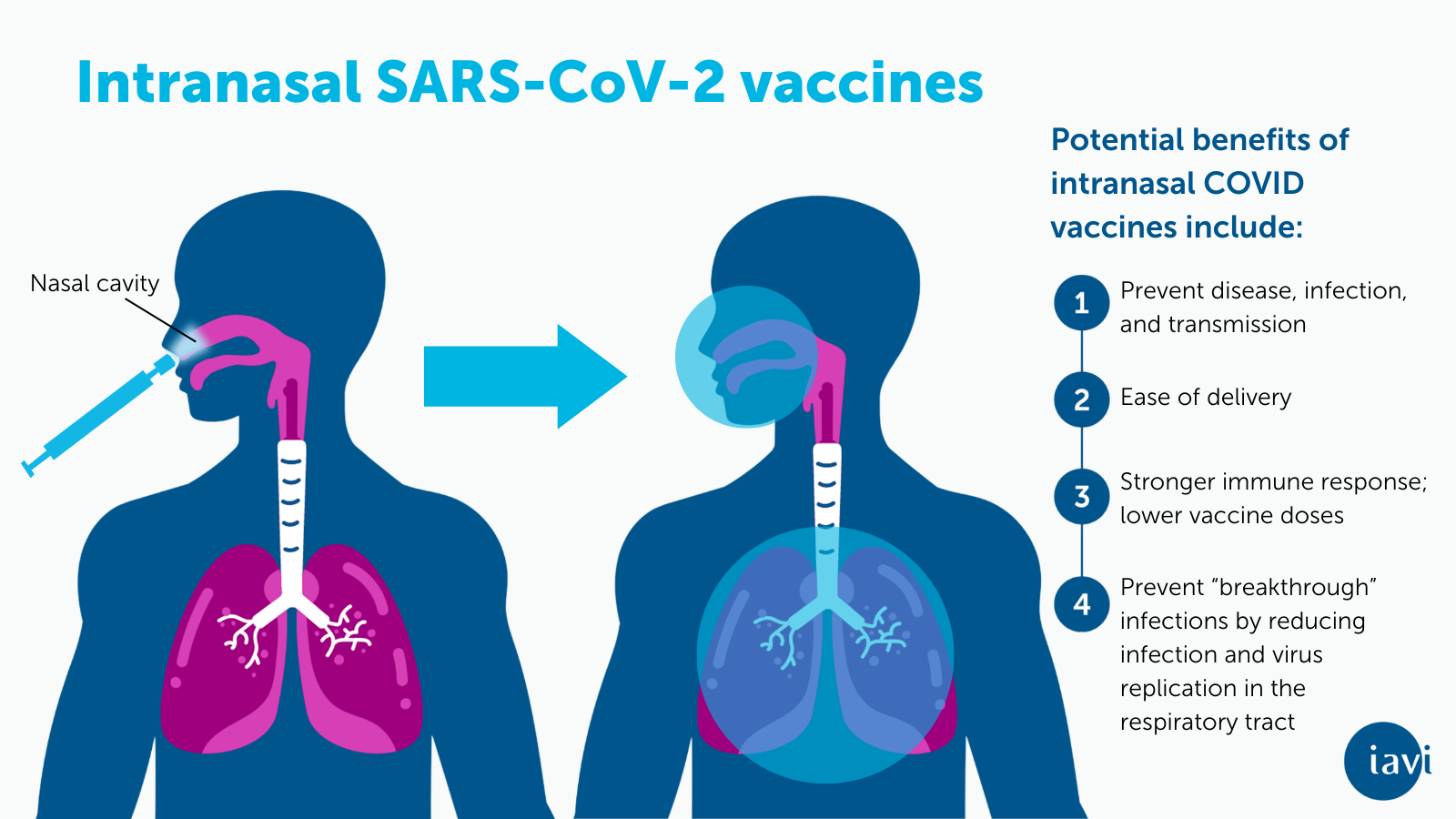
In the same study of IAVI’s rVSV-based COVID-19 vaccine candidate (also called rVSV-SARS-CoV-2), scientists also compared intramuscular injection of rVSV-SARS-CoV-2 to mucosal delivery and found that intranasal + oral-mucosal delivery induced immunity that significantly reduced SARS-CoV-2 infection and virus replication in the respiratory tract of hamsters when compared to the injectable form. Together, these experimental findings suggest that mucosal vaccination may prevent SARS-CoV-2 replication in the lungs and reduce infection in the upper respiratory tract potentially protecting from serious illness while also controlling milder infections that still contribute to virus transmission. If mild upper respiratory tract infections can be prevented, our ability to combat COVID-19 will greatly increase. These preclinical results suggest the possibility that a rVSV-based mucosal vaccine can prevent even upper respiratory tract infections not stopped by current COVID-19 vaccines.
This research serves as the foundation for pursuing human clinical trials to evaluate the safety, immunogenicity, and efficacy of IAVI’s intranasal VSV-SARS-CoV-2 vaccine in a field that needs transmission-blocking vaccines. Currently, there are more than 100 oral or nasal (mucosal) SARS-CoV-2 vaccine candidates in development around the world and two vaccines have been approved for use in China and India. This paper adds to the body of evidence for further development of mucosal vaccine candidates, which scientists believe have the potential to provide rapid and localized protection (read more in Nature).
IAVI is grateful for our partnerships with the U.S. Defense Threat Reduction Agency, the Biomedical Advanced Research and Development Authority, and Merck & Co., Inc. Kenilworth, NJ, USA (known as MSD outside the USA and Canada). Without these partners this research would not have been possible.
Read the full paper in eBioMedicine :
Espeseth AS, Yuan M, Citron M, Reiserova L, Morrow G, Wilson A, et al. Preclinical immunogenicity and efficacy of a candidate COVID-19 vaccine based on a vesicular stomatitis virus-SARS-CoV-2 chimera. EBioMedicine. 2022 Aug 1;82:104203.