December 20, 2021
The global stakes for vaccine access
COVID-19 has made stark inequities in global access to vaccines, drugs, and diagnostics more visible and alarming than ever.
Michael Dumiak
After nearly two years of a pandemic propelled by a novel and deadly airborne pathogen — for which vaccines were developed at astounding speed — agreements are only now in place to manufacture the new vaccines on the African continent.
On an optimistic timetable, construction will start on plants capable of manufacturing mRNA-based COVID-19 vaccines and other products in the middle of 2022. It’s not clear when actual production will start.
This comes as the world’s poorest countries struggle to obtain vaccine supply. Out of 5.5 billion doses of COVID-19 vaccines delivered, only 0.6% have gone to low-income countries. Only a quarter of African nations have enough vaccine to reach 10% of their populations. The goal of distributing two billion COVID vaccines to developing countries this year will fall short by perhaps 500 million shots. And in some countries where vaccines are available, it has proven challenging to distribute them quickly. As of now, more booster shots have been administered in high-income countries than the total number of first doses distributed to low-income countries, an imbalance drawing sharp criticism.
This was a running topic at the recent World Health Summit in Berlin, which brought together public health officials, pharmaceutical company executives, researchers, and representatives from the World Bank and the World Health Organization (WHO). “It’s an honor to be here, but I also seriously considered not coming,” said Ayoade Alakija, co-chair of the African Union’s COVID-19 African Vaccine Delivery Alliance. She addressed a panel in Berlin launching the annual report for the Global Preparedness Monitoring Board, a pandemic preparedness watchdog set up in the wake of the 2014-15 Ebola epidemic.
“It is inequity in the system that we don’t have many people here from the global south because, quite frankly, they can’t get here. They don’t have the vaccine passports. They don’t have the ability to test. They don’t have the ability to navigate the system,” said Alakija. With emotion, she described a divided world, hinging on the question of access. “It’s not that we don’t have vaccines that we can share. It’s not that we don’t have therapeutics. It’s not that we don’t have the tools. It’s that we choose not to act,” she added. “We have created a totally divided world, within an already divided world.”
This was a few weeks before the new Omicron variant of SARS-CoV-2 was identified by South African scientists, causing several countries to shut down travel connections to southern African nations, with Japan and Israel both closing down all international flights.
The fight over access isn’t new, but its stark inequity was made acutely visible with the COVID-19 pandemic, which has killed 5 million people and infected 252 million. Tedros Ghebreyesus, head of the WHO, considers vaccine equity the challenge of our time. “One we are failing,” he told a ministerial meeting earlier this year.
Ensuring faster more equitable access will require overcoming obstacles in development, production, and financing and distribution of vaccines, therapeutics, and diagnostics. These obstacles are substantial. But public health experts insist they can and must be solved, not just for COVID-19 but for long-running epidemics like tuberculosis and HIV, and for the daunting prospect of future emerging pathogens.
From sequencing the novel SARS-CoV-2 virus in January 2020 to emergency use of new vaccines less than a year later, the astounding pace and success of COVID-19 vaccine development is by now well documented. But access lags far behind the science.
Peter Hotez, dean of Baylor College of Medicine’s National School of Tropical Medicine, says vaccine access, and not just efficacy, needs to be considered from the very beginning of the vaccine development effort. For some time now Hotez has backed vaccine designs that can be used in resource-poor settings, where most of the world’s population lives.
In the crisis of the current pandemic the emphasis has been on speed and innovation, and it produced some of the most effective vaccines we have now: the Pfizer/BioNTech and Moderna mRNA-based COVID-19 vaccines, and the AstraZeneca/Oxford and Janssen/Johnson & Johnson adenovirus vector-based shots.
But vaccinating the world against COVID-19 requires billions of doses, and now additional booster doses on top of that. “As any engineer can tell you, with new technology there is a learning curve. We simply cannot go from zero to six or nine billion doses with mRNA and adenovirus-vectored vaccine,” Hotez says. “Innovation is great, and you can rapidly immunize millions of people with mRNA vaccines and adenovirus vaccines. But to scale it to the nine-billion level, it’s necessary to balance the portfolio with some old-school vaccines that we know we can scale up now.”
To do this, Hotez and his colleagues raised millions in private financing to fund development of their protein-based antigen, developed by the Texas Children’s Hospital Center for Vaccine Development at Baylor, for which he and Baylor microbiologist Maria Elena Bottazzi are co-directors. The vaccine is in advanced clinical trials in India, where it is known as Corbevax (the technology is already also licensed to developers in Indonesia and Bangladesh with a different name). It is a recombinant protein vaccine employing the SARS-CoV-2 spike receptor binding domain: in India the antigen is formulated in aluminum hydroxide and coupled with a Dynavax TLR-9 agonist adjuvant.
Hotez hopes this candidate will be available soon for emergency use in India and Indonesia. Baylor has already transferred the technology to Biological E, a vaccine manufacturer in Hyderabad, India, and facilities are being prepared to produce 100 million doses a month if it is authorized for emergency use, with an advance purchase of 300 million doses from the Indian government. Hotez describes this approach as a “people’s vaccine,” and says there are ongoing tech transfers in process with multiple other Asian and African countries.
Jason McLellan at the University of Texas in Austin and colleagues at the nonprofit PATH Center for Vaccine Innovation and Access have also collaborated on a pathway to a cheaper and more easily produced and distributed COVID-19 vaccine. This one can be manufactured in chicken eggs, similarly to the way flu shots are made and using the same manufacturing facilities. This candidate employs McClellan’s technique for stabilizing the SARS-CoV-2 spike protein antigen along with a viral vector used by researchers at the Icahn School of Medicine at Mount Sinai for multiplying the antigen in chicken eggs. It’s been reported that a single egg could yield five to 10 doses of COVID-19 vaccine. Clinical safety trials concluded successfully in September and Phase II trials are now ongoing.
Another protein-based candidate from the U.S. company Novavax is already in use in Indonesia and the Philippines and is currently under review by European regulators. This candidate is reported to have 89.7% efficacy against COVID-19, with higher rates against the original virus strain.
Mark Dybul, chief executive of Enochian BioSciences, former director of the Global Fund to Fight AIDS, Tuberculosis and Malaria, and an IAVI board member, says additional vaccines are one way to ensure broader access and will be needed in the future, both for SARS-CoV-2 variants and emerging pathogens. But he doesn’t see why new technologies — like the mRNA platform — can’t be instead made to work more broadly around the world.
“You could do protein-based vaccines, but why?” he questions, when the mRNA technologies offer flexibility and are highly effective. “We’re going to need new vaccines anyway, so why not invest in those systems. Then we’ll have them available for the next virus.”
Dybul says vaccine production should occur “all over the world in a decentralized way,” even for mRNA vaccines, by using manufacturing facilities with modular closed-system bioreactors. These employ single-use components for sterile processing, thereby saving on fixed costs and providing security against contamination.
A recent New York Times report suggests there are 10 potential mRNA manufacturers in low- and middle-income countries, none of which are ready to start producing vaccine right now. And timing is an issue.
COVID-19 continues to circulate unabated in large numbers of unvaccinated people in low-and middle-income countries (LMICs): this is at a terrible human cost, but also gives the virus more of a chance to evolve, giving rise to new variants. Currently more than 303 million doses have been distributed from wealthier countries to LMICs, with the U.S. alone pledging to buy and donate another 1.1 billion mRNA shots. They are starting to ship but the logistics are daunting and the ultra-cold chain required for storing mRNA-based shots is a big challenge. China says it will donate 600 million doses of its vaccines, with another 400 million to be made in joint production between Chinese companies and African countries.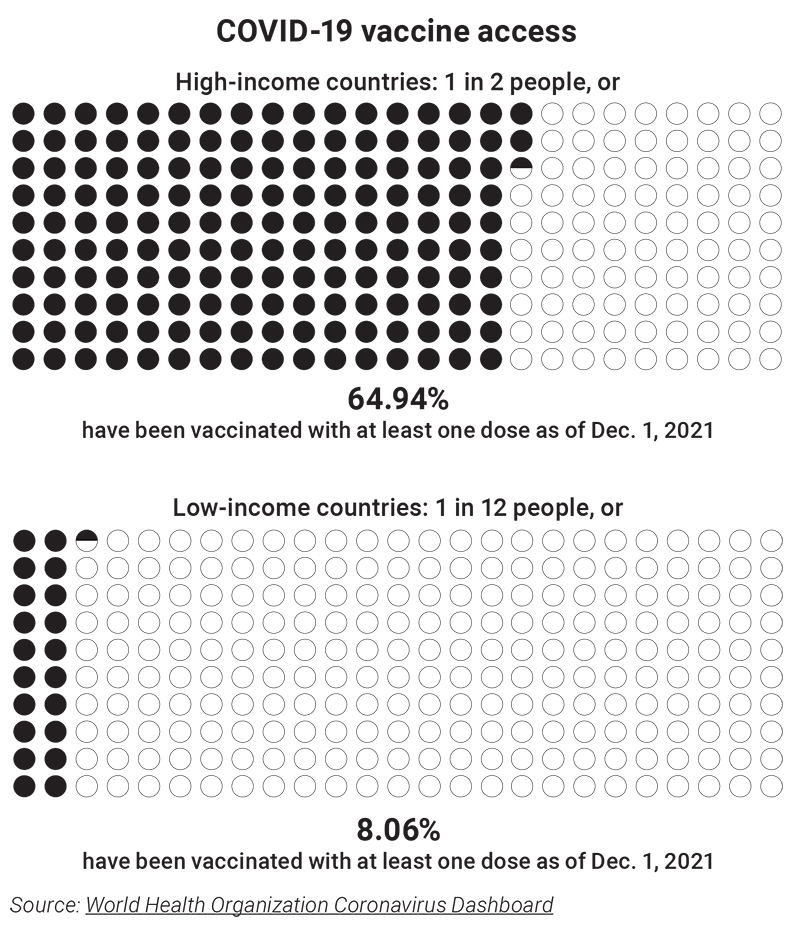
“Here in the United States and in Europe the discourse is primarily focused on giving additional boosts to people who are fully vaccinated. While that probably is beneficial, when 7% of Africa is vaccinated, the health disparities are stark,” says Dan Barouch, who directs a virology and vaccine research program at the Harvard Medical School’s Beth Israel Deaconness Medical Center and at the Ragon Institute of Massachusetts General Hospital, MIT and Harvard. “We need more vaccines to go to Africa and some vaccine platforms are just going to be better at it than others. I think the vectors and the proteins are going to be much better at getting to remote areas in Africa because they don’t require a frozen cold chain.”
As SARS-CoV-2 emerged, the Coalition for Emerging Pandemic Innovations (CEPI) divided vaccine development in the face of the pandemic into four quadrants: speed, cost, scalability, and stability. “It’s fair to say you cannot do everything quickly,” CEPI’s head of vaccine research and development, Melanie Saville, said in describing these choices at the Berlin meeting. She said that when the COVID-19 pandemic began, there wasn’t time to think about thermal stability and related implications of rollout, particularly in some LMICs. “That is something that needs some significant work in order to make vaccines viable for a lot of lower- and middle-income countries, together with the need to reduce cost.”
CEPI, which launched in the wake of the West African Ebola epidemic, is charged with forward thinking as part of its remit. Even while grappling with the ongoing waves of COVID-19, it has an eye to a potentially scary future. The organization is now promoting a 100-day target plan for novel vaccine development in future health emergencies as part of its ongoing US$3.5 billion funding drive.
The idea is to compress timelines and enable an effective response in under 100 days to a novel pathogen — the next Disease X — and to rank vaccine prototypes in terms of cost, thermostability, and manufacturing ease to determine which of these can be built quickly and deployed broadly when need arises. It now also explicitly links research and access. Making sure vaccines and other countermeasures are developed that meet the needs of all segments, of all populations, in all geographies, will now be part of the organization’s core mission.
But that won’t be easy. Financing the purchase and distribution of these vaccines will likely remain an issue long after COVID-19. Many lessons will likely be learned from the COVAX vaccine distribution facility that is meant to be the global pool for purchasing and distributing SARS-CoV-2 vaccines. COVAX was the brainchild of CEPI chief executive Richard Hatchett and Seth Berkley, the head of GAVI, the Vaccine Alliance (and former head of IAVI; see The past, present, and future of IAVI).
COVAX is administered by GAVI, CEPI, and the WHO, with support in supply and distribution from UNICEF (the United Nations Children’s Fund). The idea was to pool COVID-19 vaccine purchases under a single umbrella where high-income countries would funnel at least part of their vaccine purchases. That would give COVAX the means to negotiate bulk vaccine purchases from pharma manufacturers: participating payers in COVAX would gain some leverage in bidding for doses, while another arm of COVAX would subsidize shots and funnel them to 92 LMICs with the goal of vaccinating 20% of those populations by the end of 2021. Novel Coronavirus SARS-CoV-2. This scanning electron microscope image shows SARS-CoV-2 (round blue objects) emerging from the surface of cells cultured in the lab. SARS-CoV-2, also known as 2019-nCoV, is the virus that causes COVID-19. The virus shown was isolated from a patient in the U.S.
Novel Coronavirus SARS-CoV-2. This scanning electron microscope image shows SARS-CoV-2 (round blue objects) emerging from the surface of cells cultured in the lab. SARS-CoV-2, also known as 2019-nCoV, is the virus that causes COVID-19. The virus shown was isolated from a patient in the U.S.
Credit: NIAID-RML
It has not been a smooth ride. Wealthy countries were able to pre-order vaccine doses and pay for them, at risk, long before anyone knew if or how effective the different vaccines would prove to be. Those orders were vast. In some cases, countries purchased three times the number of doses they would need to vaccinate their entire population. COVAX funding also came in slowly as events accelerated.
Like Hotez hopes to with the Baylor vaccine, COVAX turned to Indian manufacturers to secure large amounts of future supply: a move that looked to be logical, as India has vast vaccine manufacturing capabilities, and the country was doing better in weathering the pandemic in early 2021. Until suddenly it was not. When India succumbed to a terrible pandemic wave in April, New Delhi banned exports of shots in order to cope with the virus at home. This put a huge dent in COVAX supply that it is only now recovering from. Even poorer nations scrambled to set up bilateral deals outside COVAX; an arrangement with China is one of the first steps Ecuador took to being one of the most vaccinated nations in the region.
Public health experts agree that COVAX — or something like it — is vital to ensure equitable and timely access to public health interventions, while at the same time, there are glaring flaws in the results produced by the current system. It has a chorus of critics. Financing and producing global vaccines were prime subjects at the recent special World Health Assembly, weighted by heavy disapproval of donor-funded financing — even though there’s no clear vision for alternatives. Another line of criticism is that when massive public monies underwrite risky initial vaccine development, those funds should come with contract-bound measures to ensure better access.
A bevy of voices are still calling for intellectual property to be shared and patents and licenses to be lifted on COVID-19 vaccine technologies. But even if the strings on mRNA vaccines were as free as Linux’s systems or Hotez’s proteins, manufacturing would remain a vexing issue. It takes more than a free-use recipe to churn out vaccine in bulk — manufacturing at industrial scale is incredibly complex.
At the Berlin meeting, Rino Rappuoli, GlaxoSmithKline’s chief scientist and head of external research and development, was thinking big, outlining robotic workstations in remote parts of the world for vaccine assembly. For now, nascent production facilities in Senegal and Rwanda may be a start.
Germany’s BioNTech, developer of the antigen and mRNA construction used in the COVID-19 vaccine produced along with Pfizer, inked agreements with the Rwandan government and Senegal’s Institute Pasteur de Dakar to construct and eventually handover turnkey manufacturing lines for mRNA-based vaccine doses. BioNTech will staff, own, and operate the facility to start with, but plans to eventually transfer the capacities and know-how to local partners. The company is also in talks to expand its partnership with Cape Town-based Biovac, which is part of the Pfizer-BioNTech COVID-19 vaccine manufacturing network.
Some are critical of the BioNTech agreements with Rwanda and Senegal because they say intellectual property issues will persist even if the buildings are in lower-income nations. But Dybul is cheered by this type of investment because it’s not just based on a single vaccine, but for the mRNA technologies themselves. “We tend to invest only in the product, not in the production technology,” Dybul says. But there is potential to create technology hubs in Africa, Asia, and Latin America. “It would totally disrupt our current sclerotic approach to research and development.”
And it should be possible to make these products at lower cost, too. Dybul says there’s much to learn from experience in the HIV epidemic. Access issues still loom large there, but the global situation is dramatically improved. “We’re actually at affordable products. But it took many years to get there,” he says. “I don’t know how sustainable it is because it’s heavily dependent on external resources. But we do have widespread access. It’s not universal, but it’s pretty available.”
A turning point in the HIV pandemic came with low-cost antiretroviral treatment, much of which came courtesy of large-scale generic manufacturing. As of now this seems one of the promising and proven paths to access.
Yet even the HIV field is likely to keep facing obstacles to access. Emphasis in the future may shift to newer technologies and preventives, where access pathways are less clear. “We don’t have widespread access to PrEP [pre-exposure prophylaxis]. We certainly wouldn’t have widespread access to novel technologies,” Dybul says. “There are going to be new approaches, including cell and gene-based therapies. If we start working on it now, by the time we have them we could actually already have R&D and production capacity.”
Ayesha Sitlani, associate vice president of antibody strategy at IAVI, is encouraged by advances in monoclonal antibody research and the prospect of using them as an HIV prevention approach, work IAVI is deeply involved in. And she and others at IAVI are planning for access to these potential products now, long before they’ve been proven to work. “The same access principles of acceptability, price, and the health care systems that allow delivery — all of those aspects need to be front and center of our minds,” she says.
These problems won’t be solved quickly. But global access to vaccines, diagnostics, and therapeutics is definitely at the fore in a way it was not before COVID-19. Pharmaceutical companies are also paying attention: after Merck and Pfizer recently developed oral antiviral drugs against COVID-19 they took steps to ensure equitable access (even though wealthier countries have already also put in advance orders for the pills). Ahead of authorization they are granting royalty-free licenses to the UN’s Medicines Patent Pool (MPP), a United Nations-backed agency which issues sub-licenses to certified generic manufacturers to make and market their own versions. The MPP has agreements with several HIV antiretroviral patent-holders, as well as agreements pertaining to a tuberculosis drug and hepatitis C antivirals.
Patent and technology-sharing platforms aren’t always the answer, however, as shown by the WHO’s COVID Technology Access Pool (C-TAP). Launched in May 2020 to foster open sharing of COVID-19 product-making know-how and data, no vaccine developers participated in it.
Industry, research organizations, and public-health bodies are also considering other strategies to improve access, including tiered pricing structures. None of these approaches on their own will likely be the answer, but as the COVID-19 pandemic grinds on, and new variants such as Omicron continue to emerge, the world is being forced to reconsider the consequences of inequitable distribution of vaccines and therapies.
Michael Dumiak, based in Berlin, reports on global science, public health, and technology.