June 29, 2021
A step in the right direction
Researchers describe the experimental HIV vaccine approach called germline targeting as “shepherding” the immune system. They hope it will lead to greener pastures.
Michael Dumiak
This August a clinical trial testing an experimental HIV vaccine delivered using Moderna’s mRNA system should begin in the U.S. It will be quickly followed in September by a similar trial in South Africa and Rwanda. Harnessing the power of mRNA for HIV vaccine delivery has long drawn interest, but even more so now that mRNA rocketed to prominence with its successful deployment in COVID-19 vaccines.
These trials will be the first in-human studies using an mRNA delivery system for an HIV vaccine strategy called germline targeting — an approach some see as one of the more promising now in development. “It’s a wonderful and fascinating insight into the immune system and how it initiates lineage,” says Peter Kwong, chief of the structural biology section of the Vaccine Research Center (VRC) at the National Institute of Allergy and Infectious Diseases.
Germline targeting is the term scientists use to describe the process of guiding the immune system, step by step, to induce antibodies that can counteract HIV. As the past 40 years of effort show, this is incredibly difficult to achieve. With COVID vaccines, researchers worry about the vaccine being able to fend off a handful of variants that have become particularly worrisome. But for HIV, there are millions and millions of different viruses that have resulted from the virus’s stealth ability to rapidly mutate its Envelope protein, or Env. It is this astonishing level of diversity that any HIV vaccine must contend with.
To address HIV’s variability, researchers are pursuing vaccines that can induce so-called broadly neutralizing antibodies (bnAbs). These antibodies appear to be able to fend off most if not all HIV isolates in circulation. Developing a vaccine to induce them has meant finding the rare HIV-infected individuals who make bnAbs against the virus, studying these antibodies to see how they interact with specific parts or molecular protein regions of HIV, and then using that information to rationally design — or engineer — vaccine candidates that could elicit those specific antibodies. Over the past 12 years, scientists have been able to isolate, study, and manufacture hundreds of bnAbs, some of which, in animal studies, show the capacity to protect against infection.
The problem is that these antibodies aren’t readily generated. It takes some coaxing. Actually, a lot of coaxing. That’s where germline targeting comes in.
Germline targeting begins with a primer vaccine that activates B cells that have the potential to generate bnAbs. The goal is then to use a series of different vaccine immunogens, each more specific than the last, to nudge these B cells along until they are capable of achieving the desired result: broad and potent bnAb responses that can, theoretically, protect against HIV infection.
If this process sounds difficult, that’s because it is. But researchers are encouraged by results from an early-stage clinical trial known as IAVI G001 that shows that the initial step of the germline targeting approach can work. William Schief, an immunologist at Scripps Research and executive director of vaccine design at IAVI’s Neutralizing Antibody Center, developed the priming immunogen that was tested in this Phase I trial: an engineered protein called eOD-GT8 60mer. He presented results from the study earlier this year. Although he declined to comment for this article, others shared their enthusiasm about this early finding. “It’s a delightful result,” says Lawrence Corey, professor of medicine at the University of Washington and principal investigator of the HIV Vaccine Trials Network (HVTN). “Having this kind of start is really wonderful.”
For germline targeting to work at all, researchers need the priming immunogen to target specific naïve B cells. A large part of human immunity is made up of B cells that circulate in blood — perhaps as many as 10 billion of them in total. These B cells are on patrol against invading pathogens.
About two-thirds of the B cells found in blood are naïve B cells. When these B cells come in contact with a foreign pathogen, they travel to germinal centers where they undergo a process called somatic hypermutation. This is the process by which the immune system fine-tunes its responses against a specific pathogen. These hypermutations become a kind of training: it makes the B cells produce better and better antibodies that are more efficient and effective at binding to their targets, which is especially important for HIV. Ultimately, these mature B cells turn into plasma cells that secrete the protective antibody.
In germline targeting, vaccine researchers want to harness the machinery that allows the body to produce better and better antibodies over time to train the immune system to do it against HIV prior to exposure. The immunogen tested in IAVI G001 is focused on inducing a particular class of bnAbs that target the CD4 binding site where HIV docks to and infects human cells. There may be perhaps only one in every 300,000 naïve B cells that even have the potential to induce this type of antibody. The eOD-GT8 60mer is designed to activate and expand those cells and to persuade them to go into germinal centers, where the initial process of somatic hypermutation begins.
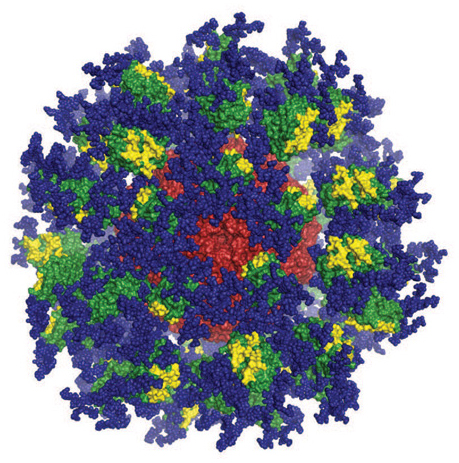 Computer image of the eOD-GT8 immune-stimulating protein. Image courtesy of Joseph Jardine, Sergey Menis, and William Schief of Scripps Research and IAVI.
Computer image of the eOD-GT8 immune-stimulating protein. Image courtesy of Joseph Jardine, Sergey Menis, and William Schief of Scripps Research and IAVI.
The next steps in the germline strategy would be to deploy one or more booster shots with different proteins strategically designed to bind and further activate that pool of memory B cells, and keep it moving it the right direction, a process Schief calls ‘shepherding.’ Then the last step in the vaccine regimen would deliver an immunogen optimized to trigger the most mature version of memory cells and convert them strongly into plasma cells that will secrete bnAbs for a very long time.
At an online conference hosted by Moderna in April, Schief described the approach like this: “You’re going to give multiple shots, with multiple different antigens in each shot, and you’re trying to basically direct the immune system on a path that you’ve predefined.”
In presenting results from IAVI G001, Schief said that ultimately the germline targeting approach would need to induce two or three different classes of bnAbs to be effective against HIV. The results from the Antibody Mediated Prevention (AMP) trials, which tested intravenous infusion of a single CD4 binding site-targeting bnAb (VRC01), also suggest a vaccine may need to induce a more robust and diverse antibody response than previously thought.
IAVI G001 shows that it’s at least possible to design a priming immunogen that can activate the specific kind of naïve B cells researchers seek. This is a key step in what are still early days for the germline targeting concept. “If you can’t get that to work, the whole thing isn’t going to work. Consistent priming of broadly neutralizing antibody precursors is a vaccine requirement. You’ve got to get it to work very, very efficiently. Otherwise, the person who you vaccinated is not going to make a bnAb later on,” Schief said.
The VRC’s Kwong is also exploring whether an engineered immunogen can induce bnAbs against HIV. His group has developed a fusion peptide immunogen, which is a sequence of amino acids that make up the engine that HIV uses to enter a host cell.
Kwong describes his approach as epitope-based, which differs from Schief’s germline-focused strategy. Kwong’s epitope-based formulations consist of just a few components: a prime of the fusion peptide immunogen and a booster with a soluble HIV Env trimer protein, known as BG505.SOSIP, along with assisting adjuvants. The goal is to induce several different lineages of bnAbs, rather than bnAbs from a single class. All of those bnAb lineages would then target one specific epitope on HIV that it uses to fuse with its target cells.
“We start first with a peptide that teaches antibody lineages to get very good affinity for just that epitope. By boosting with a trimer, we can expand the lineages and get broader responses,” Kwong says.
Experiments with the SOSIP trimer alone did not induce bnAbs in human studies, Kwong says. His group has seen positive results with the fusion peptide in preclinical animal studies, but whether it performs in humans — and with powerful enough of a neutralizing response — remains to be seen. “This is important, because we’ve just learned from the AMP study that you have to have pretty potent responses. While the fusion peptide responses we get are very broad, it’s unclear whether we get high enough potency to protect humans.”
Researchers plan to test the fusion peptide with a new adjuvant made from lipid particles and carbomer homopolymer — an electrolyte which is sometimes used to suspend solids in liquid cosmetics — in clinical trials later this year.
These structure-based strategies are complex, but some see them as the most promising route to a broadly effective HIV vaccine. The all-absorbing global health response to the ongoing COVID-19 pandemic may have obscured it from view but following last year’s failure of its latest large efficacy trial, HVTN 702, the HIV vaccine field is once again absorbing the lessons of a disappointing result. The HVTN 702 trial in South Africa was part of a broader program defining what might be needed for a successful HIV vaccine, according to a recent viewpoint essay from Corey and Glenda Gray, co-founder of the Perinatal HIV Research Unit in Soweto and head of the South African Medical Research Council. The 702 trial was meant to corroborate the very modest vaccine efficacy observed in the RV144 trial conducted in Thailand, but with a vaccine regimen adapted to the HIV subtype most common in South Africa.
The mechanism of protection shown in the RV144 trial was thought to be non-neutralizing immune responses in which antibodies mediated elimination of virus particles and virus-infected cells by a variety of mechanisms. But the failure of the regimen in HVTN 702 to provide any protection is leading researchers to weigh whether bnAb responses — no matter how difficult they are to induce — are the best, or indeed the only way to an effective HIV vaccine.
The answer may come from the two ongoing efficacy trials, Imbokodo (HVTN 705) and Mosaico (HVTN 706). These trials, developed and led by Janssen Vaccines & Prevention, part of Johnson & Johnson’s (J&J) pharmaceutical arm, are testing a vaccine candidate consisting of an adenovirus 26 vector carrying a computationally designed mosaic immunogen. The mosaic immunogen is not thought to induce bnAbs, but rather CD8+ T-cell and non-neutralizing antibody responses.
The results of Imbokodo and Mosaico will be critical to determining whether T-cell and non-neutralizing antibody responses are capable of inducing protection against HIV, Corey says. If no positive signal emerges from these trials, Corey believes there’s little point in further efforts that are not focused on inducing bnAbs. “You’d have to have a new conceptual framework for non-neutralizing antibodies in order to move that way toward a vaccine. The path to a vaccine that has multiple neutralizing antibodies is the path where the data tell us to proceed,” he says.
But Dan Barouch sees timing as a factor. “It also depends on how quickly the neutralizing antibody approaches can move forward. As of now, there has not been any vaccine that can reliably generate broad, neutralizing antibodies in humans,” he says. Barouch runs a research program at the Harvard Medical School’s Beth Israel Deaconness Medical Center and at the Ragon Institute of Massachusetts General Hospital, MIT and Harvard. His lab designed the Janssen/J&J COVID-19 vaccine and also developed the vaccines being tested in Imbokodo and Mosaico. “People are looking at many different approaches, but apart from the J&J vaccine in Imbokodo and Mosaico, everything else is still at a very early stage. All scientifically valid strategies should continue to be pursued.”
Mark Feinberg, IAVI’s president and chief executive officer and someone with experience in mass-market vaccine development and production, is also awaiting the results of the Imbokodo/Mosaico trials before casting judgment. The idea that non-neutralizing antibodies can be protective has been somewhat controversial. There are proponents and skeptics, he says. “It’s fair to say that the results of the 702 study are an important reality check for the non-neutralizing antibody approach. Obviously, we will have to see whether the Janssen studies yield any positive efficacy signal or not,” Feinberg says. “I do think if the Janssen results are negative, then it may be the end of this era of doing large efficacy trials to identify whether a concept surrounding non-neutralizing antibodies is valid.” Or at least, he adds, in the absence of neutralizing antibodies.
Others aren’t ready to abandon the hypothesis that non-neutralizing antibody responses can ultimately be protective. Immunologist Susan Zolla-Pazner at Mt. Sinai’s Icahn School of Medicine points to multiple factors that were different between HVTN 702 and the RV144 trial, including the adjuvants, the trial populations, and the incidence of sexually transmitted diseases. “Everybody says 702 is a repeat of RV144 and it’s not even close,” she says. And, given the multiple variables between the two trials, she says it is impossible to account decisively for the different results.
Zolla-Pazner knows that (as for most science) there’s limited resources, money, and logistical capacity for pursuing clinical studies. “But I don’t believe you can, at this point, remove anything from the table in terms of what kinds of immunogens will be useful and what combinations of immune responses will be most efficacious.”
Barouch expects it will be increasingly difficult in the future to fund and organize large-scale efficacy trials for HIV vaccines regardless of the concept and strategy under study, given progress with other prevention approaches, such as long-acting drugs for PrEP (pre-exposure prophylaxis). “Other forms of HIV prevention are improving,” he says.
Kwong makes a similar point. It would be one thing if the rational, bnAb-based approaches to vaccine design had lots of clinical results to point to. But most of the promising results so far have come in animals. He recalls the move toward T-cell-based approaches in labs a decade ago. “If you go back and look at the field in 2008 or earlier, everyone was focused on T cells. There’s these different pendulums that switch in different ways,” Kwong says. “As long as no one has achieved broadly neutralizing responses, I don’t think we can say. Until you start getting protective responses, we’re still nowhere, and in order for a vaccine to be successful and work, you have to pass through many different hurdles.”
Bette Korber of the Los Alamos National Laboratory helped design the mosaic immunogen being tested in the Imbokodo and Mosaico trials. She and her colleague Will Fischer think a successful HIV vaccine may well require multiple beneficial vaccine approaches: bnAb, non-neutralizing antibody, and T-cell responses.
In the meantime, engineered vaccine candidates that are designed to induce bnAbs are showcasing some of the elegant science that is underway in an effort to develop an HIV vaccine.
Schief’s germline approach — as he himself pointed out — still faces a lot of hurdles, though it jumped a high one in order to move ahead. And a partnership with Moderna to use its mRNA technologies may help quicken the next steps. Developing and manufacturing proteins, such as that used in the IAVI G001 study, is costly and slow. The next studies later this year, including the African trial that will be conducted by African labs, will use an mRNA platform instead.
The speed and cost advantages of using mRNA means an iterative approach to testing germline targeting concepts can help researchers zero in on answers more quickly. Richard Jefferys, director of the Treatment Action Group’s basic science vaccine and cure project, says the platform seems well suited. “I think the encouraging thing about mRNA is the plug and play aspects of it,” he says. “If the idea is that you now need to test out different protein constructs to see whether they can take those B cells from one step to the next, then you have a really ideal platform for doing that because you can quickly insert a gene sequence, do the experiment, and move on if it doesn’t pan out.”
Even if germline targeting is eventually successful, it is difficult to conceive of administering a course of several shots delivering different immunogens — given that it’s proving hard enough to get individuals to take one or two shots during a global pandemic in which the pathogen spreads through the air.
Feinberg is quick to say the practical considerations matter. “I’m a big believer in the importance of having vaccines readily delivered in real-world circumstances,” he says. But he remains an optimist. “If we can solve the scientific challenges, I’m optimistic that we’ll be able to find a way to solve the practical challenges. Right now, we can’t work on the practical challenges until we know what the profile of the vaccine is.”
Michael Dumiak, based in Berlin, reports on global science, public health, and technology.