November 22, 2019
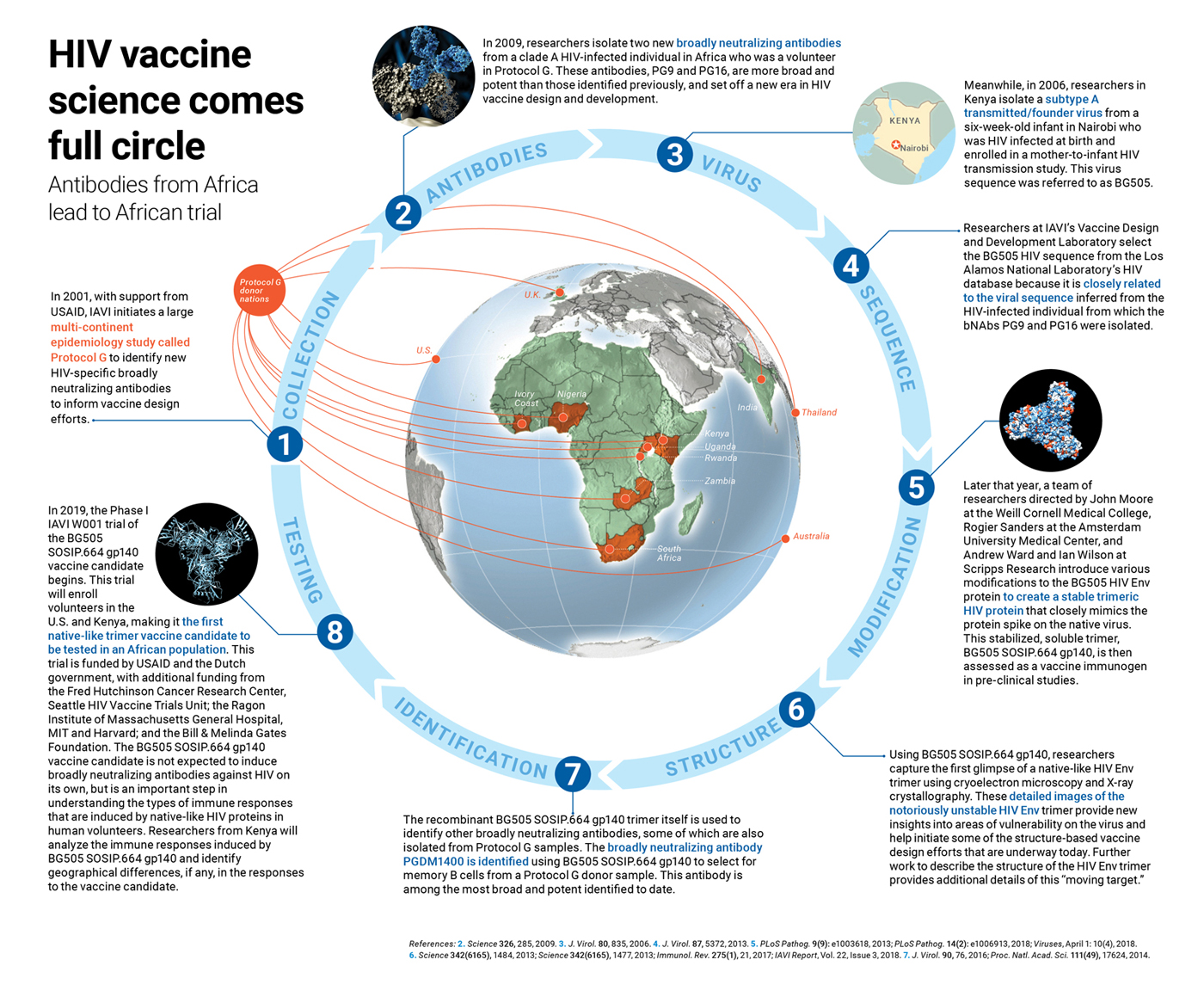
The best is yet to come
Africa is contributing far more than samples to scientific research. The continent’s scientists are actively engaged in developing and testing novel HIV vaccine candidates.
Kristen Jill Kresge
In a few months, a novel HIV vaccine trial will begin enrolling volunteers in Kenya. This trial will test whether an engineered protein that mimics the shape and structure of HIV’s outermost protein known as Envelope, or Env for short, can induce antibodies against the virus. As simple as that might sound, it took decades of work, and many failed attempts, to reach this point. And it all started with a Kenyan infant.
HIV is a notoriously difficult pathogen for many reasons. One is that the three-part or trimeric Env protein spikes that protrude from the virus’ surface are rather unstable. Given the Env protein is the target of all antibodies against the virus, this was a major stumbling block for vaccine design.
Scientists struggled for many years to sufficiently stabilize the Env trimer. Many monomeric HIV proteins were tested in vaccine trials over the years instead, but none of them induced a broadly neutralizing antibody (bNAb) response against the virus. These highly specialized antibodies that can neutralize a broad swath of HIV isolates are what many researchers predict would be the best way to protect against the diverse strains of HIV currently circulating around the globe. Scientists suspected a better mimic of the native Env trimer would be a superior vaccine immunogen, but they were largely unsuccessful in their attempts to develop one.
It wasn’t until six years ago that researchers successfully engineered a stable trimeric protein that accurately, though not exactly, mimics the native HIV Env spike (PLoS Pathog. 9(9): e1003618, 2013). This engineered trimer, dubbed BG505 SOSIP.664 gp140, was based on the HIV env gene from a clade A transmitted/founder virus—the viral variant that initiates an HIV infection—sample collected from a six-week old Kenyan infant who was involved in a mother-to-child HIV transmission study and who was infected with HIV at birth (J. Virol. 80, 835, 2006).
 The breakthrough buried in these letters
The breakthrough buried in these letters
A structural model of the BG505 SOSIP.664 HIV Env protein superimposed on the amino acid sequence of the protein (see page 3). The ribbon diagram details the positions of the beta-pleated sheets, alpha-helices, and loops that make the BG505 SOSIP.664 HIV Env protein structure. Structural model image originally published in Viruses, April 1: 10(4), 2018. Amino acid sequence originally published in PLoS Pathog. 9(9): e1003618, 2013.
The BG505 SOSIP.664 gp140 protein was tested extensively in preclinical studies by a team of scientists directed by John Moore at the Weill Cornell Medical College, Rogier Sanders at the Amsterdam University Medical Center, and Andrew Ward and Ian Wilson at Scripps Research (PLoS Pathog. 14(2): e1006913, 2018). Now, this BG505 SOSIP.664 gp140 vaccine candidate is being tested in the IAVI W001 Phase I clinical trial that already began in the U.S., and will soon begin enrolling volunteers in Kenya at the KAVI Institute of Clinical Research (KAVI-ICR) at the University of Nairobi.
And so the BG505 vaccine candidate, developed based on a virus isolated from a Kenyan infant, will return to Kenya. “Many of these products came out of African cohorts and are going back into African cohorts,” says Penny Moore, South African research chair of virus-host dynamics at the University of the Witwatersrand and the National Institute for Communicable Diseases, who studies bNAbs. “This is what all of us, all of the people who have worked on all of these things for so long, have always hoped for,” she adds.
This vaccine candidate is one of the first native-like trimer immunogens to be tested in clinical trials, and the first to be tested in an African population. But the contributions of Africans won’t stop there. Kenyan researchers will also be analyzing the antibody responses induced by the BG505 SOSIP.664 gp140 vaccine candidate, using many of the same highly sophisticated assays and tools that will be utilized by the U.S. institutions involved in the trial.
“This trial provides an opportunity to evaluate antibody responses to this vaccine in Africa, by African investigators,” says Devin Sok, director of antibody discovery and development at IAVI’s Neutralizing Antibody Center (NAC) at Scripps Research in La Jolla, CA. Sok is working closely with researchers at KAVI-ICR to validate the assays and systems for this trial. “We want African scientists to be able to lead those efforts,” he says. “We are trying to shift the focus, the mindset, and the work to Africa.”
This wasn’t always the case. Anatoli Kamali, head of IAVI’s Africa program, recalls that in the late 1990s and early aughts, some people were skeptical about the viability of HIV programs in Africa. Some experts in the field doubted it would be possible to provide comprehensive antiretroviral treatment programs or conduct high-quality HIV vaccine clinical trials in Africa. “When we started, some people still thought you could not do Phase I vaccine clinical trials in Africa,” he says.
| Researcher Spotlight: Eunice Nduati |
 To Eunice Nduati, success as a scientist isn’t dependent on geography. The factors that define success—innovative research, a solid track record of publications, and the ability to attract ample funding—are the same for scientists everywhere, including Africa. To Eunice Nduati, success as a scientist isn’t dependent on geography. The factors that define success—innovative research, a solid track record of publications, and the ability to attract ample funding—are the same for scientists everywhere, including Africa.
Nduati’s interest in understanding immune responses to infectious diseases led her to study malaria at the Kenya Medical Research Institute (KEMRI) Wellcome Trust Research Programme in Nairobi. In 2005, she enrolled for her Ph.D. training in cellular immunology under the Biology and Pathology of the Malaria Parasite/EMBL International Ph.D. program, funded by the European Commission. This allowed her to spend time between the U.K. and the coastal town of Kilifi, Kenya. After completing her Ph.D. studies, she returned to KEMRI in Kilifi to study B cells, a topic that continues to fascinate her. Nduati now has a mid-career fellowship from IDeAL (the Initiative to Develop African Research Leaders), a program based at KEMRI that aims to develop outstanding African scientists into world-class research leaders. IDeAL is one of the 11 Developing Excellence in Leadership, Training and Science (DELTAS) Africa initiatives that are a partnership between the Wellcome Trust, the U.K. Department for International Development, and the African Academy of Science’s Alliance for Accelerating Excellence in Science in Africa. Nduati is also a part of IAVI’s Vaccine Immunology Science and Technology for Africa (VISTA) consortium, which is funded by the U.S. Agency for International Development (USAID). Her work involves studying HIV-specific B- and T-cell function in the early phases of infection and determining how this impacts the subsequent quality of the antibody response against HIV. Nduati is focusing on fragment crystallizable (Fc) receptor-mediated antibody functions in particular, and is exploring whether these non-neutralizing antibody functions are associated with better viral control in infected individuals. For this work, Nduati is using samples collected from cohorts of acutely HIV-infected African volunteers who were part of IAVI’s Protocol C study. As part of this project, she spent three months at the Ragon Institute of Massachusetts General Hospital, MIT and Harvard preparing to transfer cellular assays that are needed for this type of work to her home lab in Kilifi. So far Nduati has found that very early on in HIV infection, Fc-mediated antibody responses are not significantly different in elite controllers—a small subset of HIV-infected individuals who can control viral replication without the use of antiretroviral drugs. Rather, it appears that individuals with progressive infection may develop better Fc-mediated immune responses over time. Nduati is also engaged in preparatory work for the IAVI W001 trial that is testing the safety and immunogenicity of the native-like, trimeric HIV immunogen BG505 SOSIP.664 gp140. The trial is taking place at clinical centers in Seattle and Boston, where enrollment has already begun, and at the KAVI Institute of Clinical Research (KAVI-ICR) at the University of Nairobi, where the trial is expected to start soon. Nduati is part of the team that will be exploring immune responses to the vaccine candidate, including evaluating HIV-specific memory B cells in samples collected from vaccinated volunteers from Kenya and comparing them to those observed in volunteers from the U.S. clinical trial centers. The BG505 SOSIP.664 gp140 vaccine candidate is based on a virus originally isolated from a six-week old Kenyan infant, and it will be the first engineered, trimeric HIV Env protein to be tested in Africa. “This trial shows the strength of collaboration,” says Nduati. “It also offers an opportunity to tease out potential geographical-related immune differences, if any, that are important to consider for future immunogen design.” |
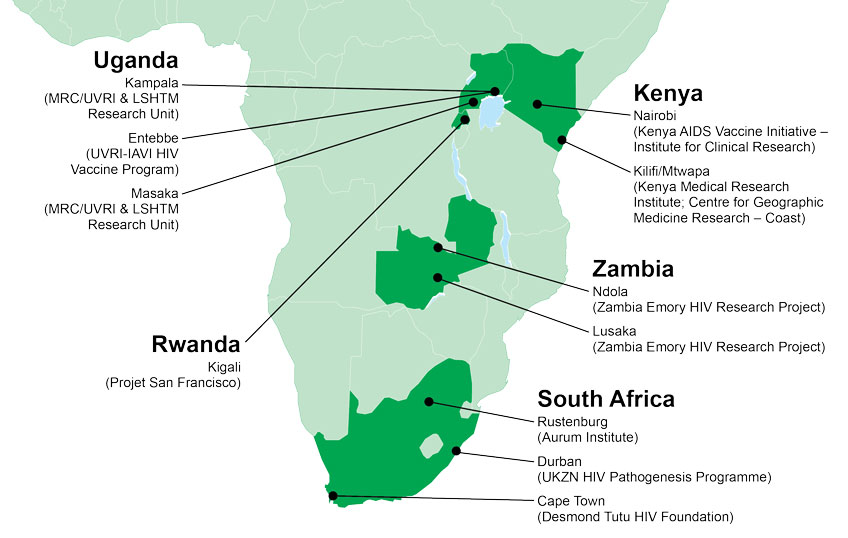
Pontiano Kaleebu, director of the Uganda Virus Research Institute (UVRI) and the Medical Research Council/UVRI Uganda Research Unit, has been working with IAVI since 2001 to develop local capacity to conduct HIV vaccine trials and has witnessed dramatic progress. “It was our plan from the beginning that we needed to build capacity locally,” he says. Uganda was the first country in Africa to conduct an HIV vaccine trial in adults and also the first to conduct a pediatric HIV vaccine trial (BMJ 324, 226, 2002). “This was critical to showing that this could happen in Africa,” adds Kaleebu.
The clinical expertise that exists in Africa today has dispelled all doubts. “The exciting thing now is African institutions and African scientists can do late-phase efficacy trials at the same standard as the north,” says Kamali.
But even after it became apparent that widespread treatment programs and gold-standard clinical trials were possible to implement and conduct in Africa, the role of African investigators was rather circumscribed. Clinical trials were often designed by researchers outside Africa, albeit with input from African principal investigators, and analysis of the trial samples and interpretation of the results was often handled by scientists in wealthier countries.
“Before, it was more about collecting samples in Africa and sending them elsewhere to be analyzed or researched,” says Vinodh Edward, chief operating officer for research at The Aurum Institute in South Africa. “Now what we are actually seeing is a number of young investigators being able to learn skills, either abroad or locally, and then being the ones who are actually doing the work on these samples. There has been a big shift and that is what excites me the most.”
This is precisely what will happen with the clinical evaluation of BG505 SOSIP.664 gp140. However, this is just one example of the increasingly important role African scientists and investigators are playing in HIV vaccine research today. Thanks to decades of investment in African institutions and investigators, there is now extensive infrastructure, a broad network of local and regional expertise, and a group of passionate and talented young researchers on the continent who are poised to contribute significantly to the goal of developing an HIV vaccine.
Eunice Nduati, a researcher at the Kenya Medical Research Institute (KEMRI) Wellcome Trust Research Programme in Kilifi, on Kenya’s coast, is one of the Kenyan researchers who will be analyzing memory B-cell responses and characterizing the neutralizing and non-neutralizing antibody responses induced by the BG505 SOSIP.664 gp140 vaccine candidate in the IAVI W001 trial. “This is an exciting time for the HIV vaccine field,” she says.
Nduati is hopeful that conducting this trial in Kenya will allow researchers to identify any geographic differences in the immune responses generated by the vaccine candidate, which can then be taken into account for the design of future vaccine candidates. “We need to have a product that hopefully will be useful where it is most needed—on the African continent,” she says.
Many governments and international organizations support genetics and public health research in Africa today. But with regard to HIV vaccines, many researchers largely credit the nearly two decades-long partnership between IAVI the U.S. Agency for International Development (USAID) with developing the capacity of African clinical research centers and investigators. “There is no other partner that has been with Africa for 15 years,” says Eduard Sanders, a principal investigator at the KEMRI Wellcome Trust Research Programme, who is supported by IAVI through USAID. “That consistency is key.”
Beyond consistency, Edward sees something unique about the collaboration between IAVI and the network of clinical research center partners it helped create with USAID funding. “In my opinion, no one besides IAVI through USAID has been able to get the science taught and translated through African scientists. USAID and IAVI had faith in the people and the sites they were developing in Africa.”
This is perhaps best illustrated by IAVI’s ADVANCE (Accelerate the Development of Vaccines and New Technologies to Combat the AIDS Epidemic) program, which is funded by the current five-year cooperative agreement between IAVI and USAID, funded through the United States President’s Emergency Plan for AIDS Relief (PEPFAR). The guiding philosophy behind ADVANCE, the latest installment of IAVI’s partnership with USAID, was to shift scientific research and leadership to Africa so that investigators there could drive the development of an HIV vaccine. “People in Africa needed to be taken seriously and given the opportunity to actually be able to do this work,” Edward says.
This includes providing young and mid-career researchers in Africa with the education, skills, and tools they need to conduct cutting-edge science on the continent. This is the goal of IAVI’s Vaccine Immunology Science and Technology for Africa (VISTA) consortium within ADVANCE, a team of international researchers including Nduati.
Today, thanks to ADVANCE, the previous cooperative agreements between IAVI and USAID, and many other efforts to develop the scientific capacity of African researchers—including those by the Wellcome Trust, the U.K. Department for International Development, the sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), the U.S. National Institutes of Health (NIH), the African Academy of Sciences, and many other governments and international funders—many say it is becoming easier for scientists to establish their careers in Africa.
“There’s just a lot more support these days for doing that,” says Tom Hope, professor of cell and molecular biology at the Feinberg School of Medicine and professor of biomedical engineering at the McCormick School of Engineering at Northwestern University, who collaborates with investigators at the University of Nairobi on mucosal immunology research.
These early career scientists are able to link into a network of established African investigators, who are already making substantial contributions to understanding the pathogenesis, virology, and immunology of HIV, as well as efforts to develop vaccines and other prevention strategies. One of these mentors is Professor Thumbi Ndung’u, program director of SANTHE.
SANTHE, one of 11 of the DELTAS (Developing Excellence in Leadership, Training and Science) Africa initiatives funded by the Wellcome Trust and the U.K. Department for International Development, is actively engaged in developing scientific talent on the African continent. Ndung’u says years of funding from IAVI allowed SANTHE to establish its research on acute HIV infection, which then helped the group secure additional funding from the Wellcome Trust, the Bill & Melinda Gates Foundation, as well as the U.S. biopharmaceutical company Gilead Sciences, which is collaborating with SANTHE on HIV cure research. With this broad base of financial support, SANTHE is training 80 young African scientists in virology, immunology, and bioinformatics, including supporting many of the young investigators who are also receiving support through IAVI’s ADVANCE program.
“We have contributed a lot to HIV vaccine research as African investigators,” says Ndung’u. “A lot of the understanding of immune responses to acute infection was done by African scientists or in collaboration with African scientists.” Ndung’u also credits African researchers with helping to define the role CD8+ T cells play in disease progression, contributing to the identification of so-called elite controllers (HIV-infected individuals whose immune systems can control the virus without antiretroviral therapy), helping determine the role human leukocyte antigen plays in controlling HIV, and making contributions to describing HIV diversity on the African continent. “African scientists have also played a key role in identifying broadly neutralizing antibodies and in conducting vaccine studies,” he says.
Ndung’u and others, including Sok, are now preparing the next generation of African researchers to continue this work. For Sok, whose experience and knowledge belies the fact that he too is a young researcher, working closely with some of the early and mid-career investigators in Africa through IAVI’s VISTA consortium is rewarding. He particularly enjoys connecting young African researchers to the broader HIV research field and exposing them to others who can rigorously challenge their ideas. “That’s the way to become a better scientist,” he says. “Our goal is not to do me-too science in Africa and India. We are trying to focus on things that haven’t been studied previously in the African context and that will contribute to the global efforts to develop an HIV vaccine.”
For VISTA, this includes conducting basic science research to help develop both bNAb and T-cell based vaccines. The group is hoping to develop and advance a conserved HIV T-cell vaccine candidate into clinical trials within the next few years. Other VISTA projects include determining how the circulating viruses in different regions have changed over time, what impact viral recombination might have on vaccine design, whether there are geographic differences in immune responses to vaccine candidates, and identifying more new bNAbs.
Hope’s team just received highly competitive grant funding from the NIH to pursue more mucosal immunology research with partners at KAVI-ICR and the University of Nairobi in Kenya, which they expect will help answer questions about some of the factors that enhance HIV transmission and dissemination.
Much of this work requires transferring technologies that were developed abroad to laboratories in Africa. Given some of the basic challenges of doing research in Africa, such as long delays in procuring reagents, this requires substantial time and resources. Scientists also have to deal with the complexity of standardizing assays across continents so the results, including those from the Phase I trial of the BG505 SOSIP.664 gp140 trimer and from future studies of even more complex vaccine candidates and immunization regimens, are comparable.
But Sok and others say this investment is well worth the effort. “They already have so much knowledge and can contribute so much, we are just providing the resources to do it locally.” And for many researchers, these cross-continent collaborations are mutually beneficial. Elise Landais, senior research scientist with IAVI’s NAC, recalls traveling to Africa and being inspired by the group of young and motivated researchers she met there. “We have so much to learn from them,” she says.
Hope also warns against assuming that partnerships with African researchers are a one-way street. “The mistake is thinking that science there is done at a different standard,” he says. “When I started at KAVI, their flow cytometry was stronger than in my lab.”
Robin Shattock, head of mucosal infection and immunity within the department of medicine at Imperial College London and the chair of ADVANCE’s scientific advisory group, thinks the entire process of vaccine development should be a partnership. “Any vaccine should become something that is jointly developed,” he says. “It should not be something that’s coming from the northern hemisphere and then being applied in an African setting.”
And on this point he gives ADVANCE high marks. “I think it’s about getting the right mix of training, opportunity, buy-in, and aspirations to make everybody feel that they are a valued part of the process, and I would say that ADVANCE is ahead of the game with that and is a good blueprint for others to follow,” he says. “For many other organizations, their default is still not to fully engage the potential of African partners. ADVANCE is a flagship approach for changing that dynamic, and what the program is trying to achieve is very laudable and clearly important.”
Although many say there is still room for improvement. “You are now seeing more publications come from Africa and more African investigators get funded directly,” says Ndung’u. “Yet, I don’t think we are there completely. Compared to our colleagues in developed countries, there are still fewer opportunities here,” he says. “But we are beginning to see a shift.”
This shift comes at a pivotal moment in the quest to develop an HIV vaccine. After decades of mostly disappointing results, researchers in the HIV vaccine field are increasingly, and somewhat uncharacteristically, optimistic. There are two ongoing vaccine efficacy trials, as well as an efficacy trial known as the antibody-mediated prevention, or AMP, study to determine whether direct administration of some of the recently isolated bNAbs are effective at preventing HIV infection. All of these trials are taking place in Africa.
| Ongoing Efficacy Trials in Africa |
| HVTN 702 (uhambo): A Phase IIb efficacy trial in South Africa testing a clade C canarypox vector-based candidate prime, ALVAC-HIV (vCP2438), followed by a genetically engineered HIV gp120 protein boost also derived from subtype C that is co-formulated with the adjuvant M59. This is a reformulated version of the only vaccine regimen to date that offered any protection against HIV infection in the RV144 trial. This trial involves 5,400 volunteers.
HVTN 703/HPTN 081: The antibody-mediated prophylaxis, or AMP, study is a Phase IIb proof-of-evaluating whether direct administration of the broadly neutralizing antibody VRC01, which was identified and developed by researchers at the Vaccine Research Center at the U.S. National Institutes of Health, can protect African women from HIV infection. This trial involves 1,900 women from Botswana, Kenya, Malawi, Mozambique, South Africa, Tanzania, and Zimbabwe. HVTN 705/HPX 2008 (IMBOKODO): An efficacy study testing a combination of two experimental vaccines to prevent HIV infection. The vaccine regimen consists of an adenovirus serotype 26 (Ad26) viral vector-based candidate that carries a tetravalent mosaic antigen as the prime, and the same vaccine along with a clade C HIV gp140 protein boost. Mosaic antigens are computationally derived to provide optimal protection against the diverse strains of HIV that are in circulation globally. The trial involves 2,600 women in Malawi, Mozambique, South Africa, Zambia, and Zimbabwe. |
“The fact that we have these massive efficacy trials underway, more than we’ve ever had simultaneously, is hugely exciting,” says Penny Moore.
There is also a new generation of vaccine candidates designed to kickstart the induction of bNAbs, including BG505 SOSIP.664 gp140, which are or will soon be in clinical trials. “We are in this era of experimental medicine in which we are starting to ask questions about our immune system and how it actually responds to all of these engineered tools that we’ve come up with that are based on incredibly elegant experiments in the lab,” she adds.
This is a much rosier picture than was the case 15 years ago. One of the major developments that brought the field to where it is today was the identification of new, more potent bNAbs. This story also begins in Africa.
In 2009, scientists at IAVI’s NAC reported on the isolation of two new bNAbs, dubbed PG9 and PG16 that were identified from clade A HIV-infected volunteer in Africa (Science 326, 285, 2009). These antibodies were the first of now hundreds of new bNAbs that have set off a new era in HIV vaccine development.
For decades, only a handful of known HIV-specific bNAbs were available. To address this gap, researchers at IAVI, in collaboration with their partner network of clinical research centers (see map, above), set out to identify others. They did this by designing a large epidemiology study known as Protocol G. In this study, researchers collected blood samples from chronically HIV-infected individuals (infected for at least three years) across several continents, and then screened their sera for broadly neutralizing activity against a panel of HIV isolates. Researchers then isolated B cells from the sera samples that could neutralize the widest variety of viral variants.
This landmark study, funded by USAID, involved 2,100 volunteers, including many in sub-Saharan Africa. It led to the isolation of the antibodies PG9 and PG16, which were identified from a single Protocol G volunteer. These antibodies exhibited a remarkable ability to neutralize a broad swath of viruses, greater than any of the bNAbs previously identified, and were also very potent.
The discovery of PG9 and PG16 also set off a cascade of other developments that helped accelerate vaccine design efforts. Isolation of these two new bNAbs were integral to identifying the BG505 HIV isolate (J. Virol. 87, 5372, 2013). Scientists from IAVI’s Vaccine Design and Development Laboratory in Brooklyn, NY, led by Simon Hoffenberg, utilized a bioinformatics approach to select the BG505 virus from the Los Alamos National Laboratory’s HIV database because of its similarities to the virus strain that infected the individual from which PG9 and PG16 were isolated. “These samples [from Protocol G] were critical to that,” says Landais. The identification of the BG505 virus isolate then led to the development of the BG505 SOSIP.664 gp140 native-like, trimeric protein that is now being tested as a vaccine candidate in the IAVI W001 trial. Other native-like trimeric HIV proteins that are now in clinical trials were also developed based on the BG505 viral sequence.
| Other trimer trials |
| Another native-like Env trimer (Trimer 4571) developed by researchers at the Vaccine Research Center at the U.S. National Institute of Allergy and Infectious Diseases is being tested in a Phase I clinical trial involving 25 volunteers in Bethesda, Maryland. This candidate is also based on the clade A BG505 viral strain (see figure, above). Another Phase I trial is testing the first combination of native-like trimeric proteins in 50 volunteers at Imperial College London. These proteins were developed by the European AIDS Vaccine Initiative (EAVI2020), a collaborative effort supported by the European Commission to accelerate the search for an effective HIV vaccine. |
The engineering of BG505 SOSIP.664 gp140 in turn led to the first cryoelectron microscopy image of a native-like HIV Env trimer bound to an antibody (Science 342(6165), 1484, 2013), as well as the first crystal structure of this stabilized trimeric protein by scientists at Scripps Research, who are also part of IAVI’s NAC, in collaboration with Sanders and John Moore (Science 342(6165), 1477, 2013). These detailed structural pictures of a native-like HIV Env trimer provided a blueprint for some of the structure-based vaccine design efforts that are underway today.
 UVRI and IAVI staff on Kiimi Island, where they are recruiting women for the HPTN084 PrEP efficacy trial.
UVRI and IAVI staff on Kiimi Island, where they are recruiting women for the HPTN084 PrEP efficacy trial.
“Neutralizing antibody research is moving along at a pace that we couldn’t have predicted 10 years ago,” says Eric Hunter, a professor at the Emory Vaccine Center and in the department of pathology and laboratory medicine at the Emory University School of Medicine, who is also a member of ADVANCE’s scientific advisory group.
The stabilized BG505 SOSIP.664 native-like trimers also led to the identification of other bNAbs, of which there are now more than 200, including others isolated from Protocol G samples (Proc. Natl. Acad. Sci. 111(49), 17624, 2014; J. Virol. 90, 76, 2016). The PGDM1400 antibody, which is among the broadest and most potent identified to date, was isolated by Sok and colleagues from a Protocol G donor sample using the BG505 native-like trimer as bait.
Researchers have since amassed detailed information about how and where many of these antibodies bind to HIV Env, and in doing so have identified multiple targets on the virus that can be exploited by for vaccine design (Retrovirology 15, 61, 2018). It turns out that nearly the entire surface of the Env trimer is targeted by one or more bNAbs (Immunol. Rev. 275, 161, 2017). “Contrary to earlier perceptions, there are not just a few areas of vulnerability on the trimer, there are many,” write John Moore and Sanders. “The human immune system has found a range of different solutions to the problem of generating bNAbs.”
Now vaccine researchers are trying to come up with their own solutions to this problem.
Another pivotal epidemiology study was launched by IAVI in 2006, also with funding from USAID. The goal of this study, known as Protocol C, was to identify recently HIV-infected individuals and to follow them over time to determine markers of disease progression. The study enrolled more than 600 individuals from nine clinical research centers in Kenya, Rwanda, South Africa, Uganda, and Zambia for long-term follow up. From this cohort, researchers were able to identify acutely HIV-infected volunteers, sometimes within days of their contracting the virus, and determine how their immune systems responded to infection.
Researchers are still mining samples collected in Protocol C to explore many questions related to natural control of the virus and to garner clues about how to design a vaccine to induce protective immune responses. To date, more than 100 scientific publications have resulted from this single epidemiology study. IAVI’s DataSpace, a critical part of the ADVANCE program, is a bioinformatics project that collects all the clinical and immunological data and biological samples collected in Protocol C and makes them available to the broader scientific community.
“We are sitting on this gold mine of samples that would be impossible to get today,” says Edward, who is also the principal investigator of IAVI’s Protocol C. “It’s highly costly following up a large cohort of people, and finding acute HIV infection is literally like finding a needle in a haystack. That’s what makes these samples so valuable.”
While the work to develop an HIV vaccine continues, investments in developing the scientific capacity in Africa are benefiting the broader field of public health research. “What HIV has brought is the understanding that research is important and that we must use what we have learned from HIV to address emerging infectious diseases and non-communicable diseases,” says Kaleebu.
The clinical capabilities to conduct HIV vaccine trials that were developed with IAVI’s support are now enabling UVRI to work in other areas of HIV prevention, including the Phase III HPTN 084 trial that is testing the long-acting antiretroviral cabotegravir for pre-exposure prophylaxis (the use of antiretrovirals to prevent HIV infection) in women at risk of HIV infection from the fishing communities surrounding Entebbe, Uganda (see photo, above). This clinical capacity is also allowing UVRI to extend its focus to other public health concerns, such as Rift Valley fever, according to Kaleebu.
This diversification is also on display at KAVI-ICR. Since becoming a clinical research institute in 2013, KAVI-ICR has been involved in clinical trials of Ebola vaccine candidates, as well as basic scientific research on newly emerging pathogens, antimicrobial resistance, and non-communicable diseases, such as cancer, with the goal of doing clinical trials of new anti-cancer molecules in the near future. “We are using what IAVI has helped us establish to work in more areas, with more diverse funding,” says Professor Omu Anzala, director of KAVI-ICR.
But even with a diversified research portfolio, one issue that concerns Anzala, Kaleebu, and almost every other African investigator you speak with is sustainability. “We rely on international funding,” Kaleebu says. “We are making efforts to increase funding from our local governments, and they have increased their funding, but not by enough.”
Now that extensive infrastructure has been built and more young scientists are being trained, the emphasis is on sustaining these advances, and identifying consistent research funding to support the careers of young scientists on the African continent. “Sustainability is a scary problem,” says Penny Moore. Kamali agrees. “Anything related to either disrupted funding or cessation of funding will have huge, huge implications.”
Penny Moore thinks the best way to ward against funding interruptions is to help young scientists become successful and confident enough to apply for and win large research grants that will sustain their work. This is part of the reason why efforts like ADVANCE, SANTHE, and Wellcome Trust fellowships are vital. They support young researchers and help them reach the point that they can apply for and receive funding from large research grant-giving institutions like the NIH. This is what happened recently for Hope and Marianne Mureithi, chief research scientist at KAVI-ICR at the University of Nairobi, and their colleagues.
This is USAID’s vision. “Some global funders who conduct research in Africa for product development see opportunity; at USAID we look at our African counterparts and see possibility,” says Margaret McCluskey, senior technical advisor for HIV vaccines at USAID. “These young, African pioneers are the future of HIV vaccinology, and we at USAID are very proud of them, and the small but important part USAID plays in facilitating their self-reliance and ultimate success.”
Mark Feinberg, president and CEO of IAVI, also sees African scientists playing a critical role in developing an HIV vaccine and recognizes the role IAVI and USAID play in helping to make this a reality. “The work being done in Africa is the starting point and the ending point,” he says. “The long-standing partnership between IAVI and USAID is enabling important scientific advances and helping build research expertise in Africa, particularly among young African scientists, who are essential for the long-term success of the AIDS response. PEPFAR, a program of unprecedented vision and impact, is facilitating this progress.”
When Edward reflects on the accomplishments of the young scientists who are part of ADVANCE (see Researcher Spotlights, above), he thinks the program is well on its way to achieving many of its goals. “If you had asked me if this would happen, I wouldn’t have believed it, but this shows what IAVI and ADVANCE have been able to achieve in just the last couple of years. If we continue this way, probably sometime between 2021 to 2026, you are going to see a lot of mind-blowing research coming out of Africa.”
Despite the many challenges, it appears that HIV vaccine research on the continent is thriving. “The goal is to bring the assets we have together to accelerate progress,” says Feinberg. “The best is yet to come.”
 In the early days of the HIV/AIDS epidemic, there was often not much a doctor could do but treat the hallmark infections that accompanied the development of AIDS. This was a time Eugene Ruzagira, assistant professor at the London School of Hygiene and Tropical Medicine (LSHTM) based at the Medical Research Council/Uganda Virus Research Institute (MRC/UVRI) and LSHTM Uganda Research Unit, recalls all too well.
In the early days of the HIV/AIDS epidemic, there was often not much a doctor could do but treat the hallmark infections that accompanied the development of AIDS. This was a time Eugene Ruzagira, assistant professor at the London School of Hygiene and Tropical Medicine (LSHTM) based at the Medical Research Council/Uganda Virus Research Institute (MRC/UVRI) and LSHTM Uganda Research Unit, recalls all too well.
 For Clive Michelo, it was never a question of if he would return to Africa. “I always planned to come back to study malaria,” he says. But when Michelo was visiting his mother in his home town of Livingstone, Zambia, he heard about an open post-doc position at the Zambia Emory HIV Research Project (ZEHRP) in Lusaka. So instead of malaria, he returned to Zambia in 2015 from his Ph.D. studies in the Netherlands and began his post-doc work at ZEHRP.
For Clive Michelo, it was never a question of if he would return to Africa. “I always planned to come back to study malaria,” he says. But when Michelo was visiting his mother in his home town of Livingstone, Zambia, he heard about an open post-doc position at the Zambia Emory HIV Research Project (ZEHRP) in Lusaka. So instead of malaria, he returned to Zambia in 2015 from his Ph.D. studies in the Netherlands and began his post-doc work at ZEHRP.
 For Sheila Balinda, her mother was, and still is, her greatest supporter. In fact, it was her mother’s suggestion that she might become a doctor that she thinks led her to pursue a career in science.
For Sheila Balinda, her mother was, and still is, her greatest supporter. In fact, it was her mother’s suggestion that she might become a doctor that she thinks led her to pursue a career in science.