May 9, 2023
Keeping it all in perspective
A view from Zambia on the shifting landscape of HIV vaccine research and the enduring optimism that characterizes one researcher’s drive.
Kristen Kresge Abboud
The last time I spoke with Clive Michelo, he had good reason to be hopeful.
Back in 2019, Michelo, a postdoctoral fellow and laboratory science lead at the Rwanda Zambia Health Research Group clinical research facility in Lusaka, Zambia, worked in an office where volunteers from the local community were coming in routinely to participate in an ongoing HIV vaccine efficacy trial known as Imbokodo, or HVTN 705. The clinic was festooned with posters explaining HIV vaccine research and introducing the trial: “An HIV Vaccine: The world’s best long-term hope for ending HIV.”
The pharmaceutical company Janssen (part of Johnson & Johnson), the U.S. National Institute of Allergy and Infectious Diseases, the Bill & Melinda Gates Foundation, and the HIV Vaccine Trials Network (HVTN) were backing this trial, testing a candidate vaccine regimen developed at Janssen that was meant to address the global diversity of HIV.
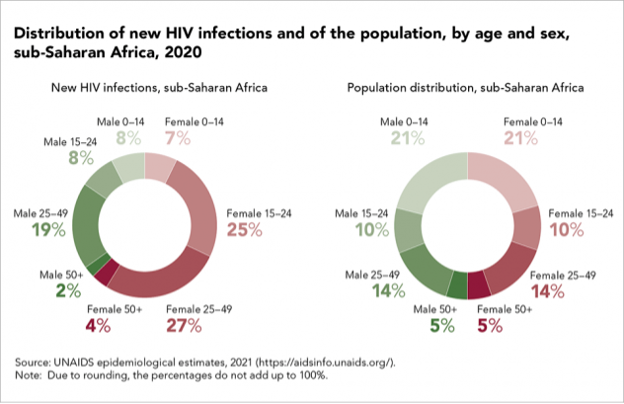
The volunteers were women at high risk of HIV infection in sub-Saharan Africa, including in Lusaka, where Michelo works.
HIV infection rates in this population remain persistently high despite advances in HIV prevention efforts and widening availability of antiretroviral therapy. According to UNAIDS, adolescent girls and young women in sub-Saharan Africa accounted for 25% of HIV infections in 2020, while only representing 10% of the population.
But in 2021, Michelo, along with the rest of the world, received some bad news. Researchers announced then that the investigational HIV vaccine regimen being tested in Imbokodo, the isiZulu word that means rock, did not protect against HIV infection.

Then, earlier this year, another HIV vaccine efficacy trial — this one known as Mosaico, or HVTN 706 — was also discontinued because a similar investigational vaccine regimen developed by Janssen was ineffective at preventing HIV infection among volunteers enrolled at more than 50 clinical trial sites in North and South America and Europe.
Along with these disappointing results, there was the COVID-19 pandemic, which included among its many negative effects, a huge disruption to clinical research worldwide.
Suffice it to say, Michelo had plenty of reasons to be discouraged.
Yet amid all these setbacks, he maintains a calm and purposeful air. He seems to have taken it all in stride and is as dedicated as ever to engaging with the community where he lives and works, and in using these negative outcomes to inform future HIV vaccine designs.
In the past few months, Michelo’s work on characterizing the early T-cell responses against HIV that are present soon after infection in volunteers from his local community was published. He also contributed to research on the importance of increasing access to laboratory-based assays to evaluate the development of drug-resistant HIV in people living with the virus in low- and middle-income countries (LMICs) to better monitor treatment outcomes.
We spoke recently over Zoom about this work and his unflagging dedication to the science of HIV vaccine research. An edited version of our conversation appears below.

Since we last spoke, two HIV vaccine efficacy trials and two trials testing antibody-medicated prevention of HIV showed no efficacy at preventing HIV infection. One of the vaccine efficacy trials took place right where you work. How did these outcomes affect you?
Well, it was disappointing. When you’re working with a big group such as the HVTN and then that happens, you have to sit back and evaluate your work a bit more critically and ask yourself why this has happened after so many years of research. And you start wondering whether it’s something that could happen to what you are working on as well. It makes you re-examine your work and re-strategize efforts to develop something that would be more efficacious.
Your research has focused in part on determining which viral epitopes should be included in a vaccine to induce T-cell responses, yet the Imbokodo trial seems to have convinced most researchers that it will likely take both T-cell responses and broadly neutralizing antibodies to fend off HIV. How does this influence your future work?
Results like this definitely re-emphasize the need for concerted efforts towards eliciting B- and T-cell responses, and these are basically the discussions we had after the results of the Imbokodo trial. The goal now is to prioritize approaches that are going to work towards an immunogen design strategy that will target both B cells and T cells at the same time. It’s clear that you will need this concerted effort in initial response towards HIV.
How did the local community respond to the news about the Imbokodo trial?
You get mixed reactions when you explain what happened. There are people who get a bit hopeful when you’re talking about a vaccine trial because to them it sounds like we’re almost there. Then, when you come back and tell them that what we were doing didn’t work, it’s disappointing. Negative news like this is disappointing to them as well as to the researchers. But right now it is really about reaching out to the community, trying to figure out their opinions about the research that is going on, and trying to just remind them that we are still working on something that we hope in the future will help us and that they will remain an important aspect of the development pipeline.
Does it make you any less confident that an HIV vaccine can be developed?
Well, I guess if you didn’t have comeback ideas and strategies, then you could probably get really stuck in disappointment. But from my end, I feel like we still have a few tricks up our sleeves that we need to try out that were still missing with the HVTN 705 and 706 trials. In a way, this kind of result tells you what not to do, so if you were going to go down that road, now you know not to, and, in a way, that gives you a bit more confidence in a strategy that is different from the failed regimen and actually makes you a bit more hopeful.
On that note, what have you found with your work on epitope mapping of immune response in the cohort of volunteers you’ve studied that could be useful to designing a future HIV vaccine immunogen?
What we’ve found is that following initial HIV infection, there are some very good responses to consensus epitopes, and there are some very good responses to a variant of those consensus epitopes. Based on this, we concluded that if you were to develop a vaccine it could be based on the global consensus antigens, but should also be complimented by variations that are representative of the target population.
Would that mean that each vaccine would then be tailored specifically to the virus circulating in that area?
That’s a bit of the unfortunate part, but we’ve kind of already been doing that for the flu vaccine. We tend to develop regional flu vaccines. What I’m envisioning is a situation where you’d have a global HIV vaccine, and then for each region, you’d augment it to something that is specific to the region just to capture a bit more of variations within the local population and improve the breadth of coverage of any vaccine modality. That is what we are envisioning the vaccine will be.
And within that design strategy, we definitely need to work together with the B-cell arm and try to come up with antigens that will elicit both B cells and T cells. We’re not sure yet whether those antigens should be delivered at the same time, or whether it might be best to start with a T-cell prime and then end up with a B-cell boost.
You also were a co-author on a study about using more lab-based assays to track and evaluate HIV drug resistance in LMICs. Tell me a bit about that.
One of the hindrances we have in monitoring people who are on antiretroviral therapy is the expertise required to run lab-based assays to monitor resistance. Also, just the cost of running these assays. So, we were working in conjunction with Catherine Kibirige at the IAVI Human Immunology Laboratory (HIL) at Imperial College London to try to develop an assay that can be cost-effective within LMIC settings and that can be easily deployed to try to improve monitoring.
Right now, when it comes to monitoring HIV antiretroviral adherence, it’s really reliant on clinical outcomes. And here in Zambia, that level of monitoring, which uses a lab-based procedure, is only available at what we call a tier-three health facility. And yet most of the people that are living with HIV are being monitored at either tier-one or tier-two health facilities. You’re only referred to a tier-three hospital if you present with obvious clinical failure, which we think is too late. We think that if these assays can be affordable and easy to do, then they could also be introduced at tier-one and tier-two facilities and that could improve HIV monitoring and care.
What is required to make this happen?
First of all, it would have to be based on standard blood samples that are collected at each site visit. Then it also has to have a quick turnaround time. Then you need to also make sure the right equipment is there, and the training has been conducted as well. And that training has to be easy and standardized and not require much expertise.
Did you, like many others, take on COVID-19-related research during the peak of the pandemic?
We did take on some COVID-related research projects. The one I was directly involved in was monitoring antibody responses to the virus and seeing if people who developed antibodies following an infection got another infection. We also tried to evaluate the prevalence of infection within the communities around us, especially the women that were coming to our site for the different studies.
What was the prevalence like at your site?
In the women that would come in for trials, we saw a gradual increase over 2020. At the beginning it was very low and we were wondering if there was some immunity within our cohorts. But then when Omicron came it just brought an abrupt increase to the prevalence within that population.
What are some of the next research projects you are taking on?
Some colleagues within the network have done T-cell epitope mapping and have found that if you use an early time point sample, so not very late after an HIV infection has occurred, the T cells tend to be very specific in the targeting of certain antigens. This is work that we are trying to replicate in Zambia now as well by looking at similar time points following infection, and basically trying to learn from natural infection.
The interesting part is that when someone gets infected with HIV, they get this high viral load and then your immune system is responsible for bringing that high viral load down to a much lower set point. In a way, you kind of establish this balance between your immune response and the virus and this is what we want to try to figure out. What is that initial immune response that is capable of bring the virus under some level of control, although not complete control? We want to see if this is something we can augment to take advantage of for a future vaccine. So, right now I’m focused on working with samples from these earlier time points.
For more about the results and repercussions of the Imbokodo and Mosaico HIV vaccine trials, see:
- Imbokodo trial results highlight challenge of developing an HIV vaccine and urgent need for access to proven prevention tools, i-base
- The Only Late-Stage HIV Vaccine Study (Mosaico) Just Tanked. What’s Next?, TheBody
- Mosaico and the Future of HIV Vaccines, A Shot in the Arm Podcast with Ben Plumley