February 15, 2023
Is stopping SARS-CoV-2 transmission an attainable goal?
Hundreds of next-generation COVID-19 vaccines are being explored, including many that are delivered mucosally to try to prevent infection or block transmission.
Kristen Kresge Abboud
In the months after the first COVID-19 vaccines were authorized, they worked remarkably well. They protected people against the most severe manifestations of the disease, as they were designed to do, but they did even more than that. Early on, mRNA-based COVID vaccines also blocked infection altogether.
This didn’t last. And the reason it didn’t last has as much to do with the virus as it does with the waning of immune responses.
“The problem is the variants,” says Robert Seder, acting chief of the Vaccine Immunology Program at the Vaccine Research Center at the U.S. National Institute of Allergy and Infectious Diseases (NIAID). As SARS-CoV-2 circulated globally, the virus acquired mutations that allowed it to escape neutralization by antibodies that were induced by the original COVID-19 vaccines. With each new variant, the vaccine-induced antibodies were less and less effective.
At the same time, neutralizing antibody titers following mRNA-based COVID-19 vaccination tapered off rather quickly. Levels of SARS-CoV-2-specific IgG (immunoglobulin G) antibodies, the most common circulating antibody type found in blood and tissues, skyrocket following intramuscular (IM) vaccination and then decline substantially.
The same dynamics are true for IgA antibodies, the type found predominantly at mucosal surfaces, which is where respiratory viruses such as SARS-CoV-2 take root. However, the IgA antibody titers induced by IM vaccination with mRNA-based COVID vaccines are much lower than IgG titers to begin with.
“Vaccination induces sky-high levels of antibody in serum, and some of that ends up at the mucosal surfaces,” according to Florian Krammer, Professor of Vaccinology at the Icahn School of Medicine at Mount Sinai. “It’s a very small fraction of what you have in serum, but when you have very high serum levels, it might be enough to protect you against infection,” he says.
But it becomes much harder to protect against infection when the antibody levels at mucosal surfaces wane, and, at the same time, new variants arise that are much less sensitive to neutralization by these antibodies. “You have 10- to 20-fold lower neutralization levels against Omicron, and even though you might have antibodies in the nose, there aren’t as many,” says Seder. Existing COVID vaccines work incredibly well to protect people against severe disease, hospitalization, and death, but they can’t block the virus entirely. Hence, the repeat infections with SARS-CoV-2 that are commonplace today.
Given this, is blocking infection or transmission still an attainable goal?
Many researchers think so. And they think the way to accomplish that goal is through mucosal vaccination.
“You could look at vaccines that could tweak the B cells to give you better antibodies, but chasing the variant is, in our view, incrementalism. I think it has to be mucosal,” says Seder. “Suppose we had a variant that transmitted like XBB or BQ.1 and caused pathogenesis — we would be back to March 2020. We need a strategy to shut this down by either preventing infection or transmission. So that’s when we had a paradigm shift that led us to mucosal immunity.”
Immunity when and where it’s needed
Mucosal vaccines are applied directly to a mucosal surface and include those that are administered intranasally, through a mist a spray, or orally, through droplets or a liquid. Many COVID-19 vaccines are being formulated for intranasal delivery.
Frances Lund, director of the University of Alabama at Birmingham Immunology Institute, is skeptical that a nasal COVID vaccine is going to block infection, but she can foresee it stopping transmission of the virus. “The power of the intranasal vaccine is reducing the amount of time that you have productive virus, which speaks directly to transmission, but also probably to the number of days you will be ill.”
Lund says that mucosal vaccination mimics what occurs in natural infection by putting immune cells, in this case memory B and T cells specific to SARS-CoV-2, in the mucosal tissues where they need to be. And even if these cells can’t block infection altogether, they can activate much more quickly. “Tissue-resident memory B cells can differentiate and start making antibody within a day. If you have a reasonable defense there [in the mucosal tissues], you’re not going to stop infection, but you are going to knock the virus down to levels that are sub-clinical much more quickly. The advantage of mucosal immunity is that it’s there at a time when the quantity of virus is relatively low,” she says.
Data from a recently published meta-analysis also suggest that mucosal immunity may help explain why hybrid immunity that is formed following both COVID-19 vaccination and natural infection is more favorable than just vaccine-induced immunity.
“If someone gets infected with SARS-CoV-2, they have a mucosal immune response. And in people who’ve had an infection, it seems that mRNA vaccines are boosting those responses to a certain degree. If you didn’t have an infection initially, then that doesn’t happen. This is why we think hybrid immune individuals are better protected,” Krammer says.
“It’s hard to prove, but mucosal immunity might be a big factor there,” he adds. Krammer and colleagues published a study last year which showed that a specific type of mucosal immune response known as secretory IgA, which is present in saliva, gets boosted in individuals who were vaccinated following natural SARS-CoV-2 infection.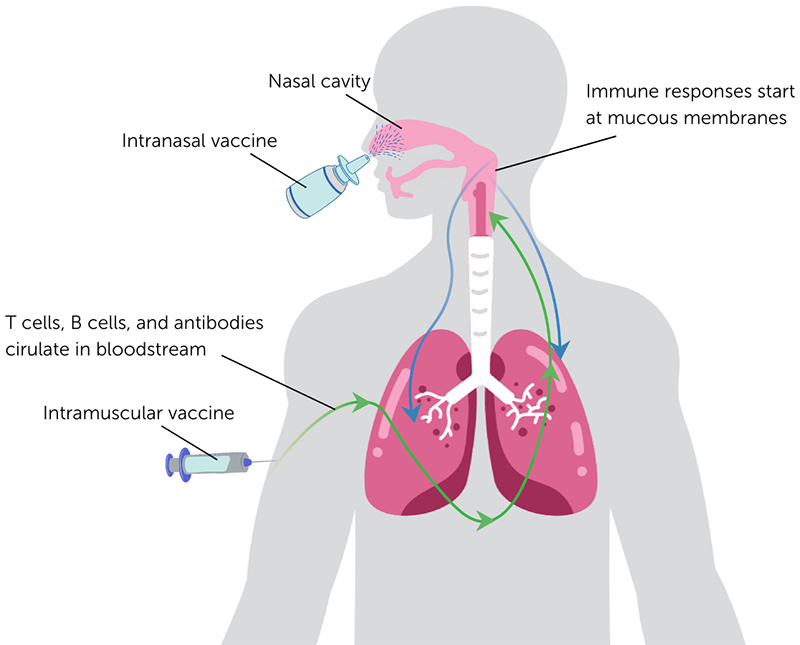 Mucosal vaccination: immunity at the site of infection. Intramuscular injection primarily induces immune responses that circulate in blood, whereas intranasal vaccination can induce more immune cells in the mucous membranes where pathogens such as SARS-CoV-2 initially establish an infection. Mucosal immune responses can help limit an infection and halt transmission of the virus to others. Researchers are exploring many different intranasal vaccine formulations against SARS-CoV-2 in an effort to curb the spread of the virus.
Mucosal vaccination: immunity at the site of infection. Intramuscular injection primarily induces immune responses that circulate in blood, whereas intranasal vaccination can induce more immune cells in the mucous membranes where pathogens such as SARS-CoV-2 initially establish an infection. Mucosal immune responses can help limit an infection and halt transmission of the virus to others. Researchers are exploring many different intranasal vaccine formulations against SARS-CoV-2 in an effort to curb the spread of the virus.
Many mucosal approaches
Krammer is one of many researchers working on an intranasal COVID vaccine to try to boost the immune responses at mucosal surfaces in attempt to thwart infection or stop transmission of the virus from person to person. A survey by Nature indicates there were approximately 100 nasally delivered vaccines for COVID in development as of September 2022, with about 20 of them in clinical trials, and one already in use.
iNCOVACC is an intranasal vaccine based on a chimpanzee adenovirus vector that was developed by Bharat Biotech and is licensed for use in India. Another mucosally administered COVID vaccine known as Convidecia Air is an inhaled SARS-CoV-2 vaccine developed by the Chinese company Cansino Biologics. It is based on an adenovirus serotype 5 vector.
Another adenovirus vaccine candidate, an intranasal formulation of Oxford/AstraZeneca’s chimpanzee adenovirus-based COVID-19 vaccine, was in development but was dropped after disappointing results in a Phase I trial.
Many other intranasal vaccine candidates in development also utilize viral vectors to deliver SARS-CoV-2 antigens. Krammer and his colleagues at CastleVax, a spinoff company from Mount Sinai Health Systems, are developing a live, attenuated Newcastle disease virus (NDV) vector-based candidate (NDV-HXP-S) that expresses the SARS-CoV-2 Spike protein and are testing it both by IM injection and intranasal administration in clinical trials.
NDV is an avian paramyxovirus that doesn’t circulate among or cause disease in humans, and Krammer says the candidate appears to have a favorable safety profile. The goal of the intranasal candidate is to induce IgA antibodies in the nose that could protect against breakthrough infections and blunt virus transmission, but Krammer says it is possible that it may also boost serum antibody levels. “Intranasal vaccines might not work well in naïve individuals in terms of giving you serum immunity, but now that almost everyone has some immunity, if you give these vectored vaccines, you may see a boost in serum antibodies too,” he explains. “If so, this could replace the IM vaccines entirely.”
IAVI is also developing an intranasal formulation of its vesicular stomatitis virus (VSV)-vectored COVID-19 candidate. The candidate was initially developed in partnership with Merck for IM administration, but this route of administration induced inferior immune responses in a Phase I trial.
Chris Parks, head of IAVI’s Vaccine Design and Development Laboratory, says the replication competent VSV vector-based candidate likely did not work by IM injection because there just weren’t enough cells in the muscle that express the ACE2 receptor that SARS-CoV-2 requires for cellular entry, which researchers realized as they learned more about the virus. The VSV candidate expresses the Spike protein on its surfaces and if it couldn’t infect cells, it likely couldn’t replicate, and therefore didn’t invoke a substantial immune response. “It seems simple but that’s probably the reason it didn’t work,” says Parks, who calls the preclinical data on intranasal administration of the VSV candidate in both hamsters and monkeys “very promising.” IAVI is now conducting safety studies in preparation for potential clinical trials in humans to evaluate the intranasal route of administration.
Researchers are also testing live-attenuated SARS-CoV-2 vaccines, protein vaccines, and even mRNA formulations intranasally. “I think it’s important to try all of the approaches,” says Lund, much as was done with the first-generation COVID vaccines.
Each approach has unique advantages and disadvantages, but many researchers agree that the most promising in terms of inducing mucosal immunity may be the live-attenuated SARS-CoV-2 vaccines, such as the one being developed by the company Codagenix, which is now in Phase III efficacy trials.
“When you think about the best vaccines we have, they tend to be live-attenuated viruses,” says Lund. “They are the most likely to induce the kind of immunity you want.” However, they have some major limitations too, as they may not be suitable for the elderly or for immunocompromised people.
Protein vaccines have been used for decades to protect against hepatitis, shingles, and other viruses and are generally considered to be safe for all populations. The big question when it comes to mucosal delivery of a protein vaccine is whether this approach will require an adjuvant to boost the immune response, and if it does, what adjuvant could be used. “People have been working for decades on what the right mucosal adjuvant is,” says Lund, but there isn’t consensus on this issue. And concerns were raised after an adjuvant used with an intranasal flu vaccine caused temporary facial paralysis.
Then there is mucosal delivery of mRNA vaccines. The mRNA vaccines developed by Pfizer/BioNTech and Moderna are the most widely used COVID-19 vaccines in the world and are the first vaccines ever licensed using the mRNA platform. Now researchers are also exploring whether these vaccines can be formulated for intranasal delivery. “They are new enough that we don’t know how great they will be,” says Lund.
Scientists from Moderna reported results in a preprint of a study testing an intranasally administered mRNA-lipid nanoparticle (LNP) encapsulated vaccine in hamsters. But this approach is not as far along as some of the others in development. “It is going to take some time to optimize the formulation of the mRNA in an LNP designed for mucosal delivery,” says Seder.
Barriers to development
One important question is how long mucosal immunity induced by any of these vaccine candidates will last. For now, researchers aren’t sure whether the durability of immune responses will be any higher than with the IM injection. “I don’t think we have any clue what the longevity of these candidates will be, and until we do the studies, we won’t know,” says Lund.
Parks says that if mucosal vaccination can induce localized, tissue-resident memory B and T cells, there is good reason to believe it should be long lasting. But there are still many questions, not only about the durability of these responses but also about where they need to be, and if intranasal vaccination will be sufficient to induce mucosal immune responses in both the nose and the oral cavity.
At a NIAID-sponsored symposium last November, Akiko Iwasaki, a professor of immunobiology and molecular, cellular, and developmental biology at Yale University, said that oral mucosal immunity will be required for a vaccine to stop transmission of the virus. Her spinoff company Xanadu Bio is developing a non-adjuvanted intranasal SARS-CoV-2 Spike protein candidate that appears promising in mice.
But promising results in rodents doesn’t necessarily mean it will work in larger animals, particularly humans. “Translation with mucosal vaccines has been an issue,” says Seder.
Beyond translation, there are also several regulatory hurdles for mucosal vaccines. There are not many licensed mucosal vaccines in the world — only the intranasal influenza vaccine known as FluMist is licensed by the U.S. Food and Drug Administration — and safety is paramount for any vaccine that is administered so close to the brain.
In addition, there are already several effective COVID-19 vaccines. “We have vaccines that work if you give them IM and so any new vaccine has to beat that or be as good,” says Lund. “The most bang you can get for your buck with an intranasal vaccine is blocking transmission and transmission studies are immensely expensive and huge,” she adds.
Krammer is hopeful that these studies, including those that enroll entire families to monitor the effect of the vaccine on onward transmission of the virus, will be done, and that the results will help inform the development of mucosal vaccines for many respiratory viruses. “I think we can learn a lot from what is being done now with these mucosal vaccines for COVID. My only worry is that there is much less funding now for doing these things and I think a lot of the development programs will just die off,” a sentiment shared by others in the field.
Seder agrees that this work will have implications far beyond the current pandemic. “If you solve it for COVID then you solve it for flu and RSV [respiratory syncytial virus]. It’s a general scientific principle,” he says. “We should’ve been doing this a while ago.”
One clear advantage of the intranasal vaccine is having a needle-less delivery system. “If you have another pandemic like we just had and you really want to vaccinate the world, this is probably a better, more scalable approach, and that matters in the long run,” Lund says.
“Even if we’re not going to end up with an intranasal vaccine for COVID, it does not mean we should not be working on intranasal vaccines. There are advantages in terms of the immunity you generate and the delivery of these vaccines,” she adds, noting that the 30 years of work on mRNA vaccines certainly paid off in our response to COVID-19. “Maybe this is what we need to do for our next platform.”
Read more about how scientists are thinking about next-generation vaccines for respiratory viruses.