July 6, 2023
Hitchhiking the mucosa
Brittany Hartwell and her lab are exploring new ways to transport vaccine antigens across mucosal barriers to trigger more protective immune responses against many pathogens, including HIV.
Kristen Kresge Abboud
Your mucosal immune system, including the mucus membranes that line your respiratory, digestive, and reproductive organs, is designed to keep things out. It acts as an important first line of defense against foreign matter of all kinds, including pathogens.
But what happens when you purposely want to shuttle something across the mucosal barrier? The answer is: it isn’t always easy.
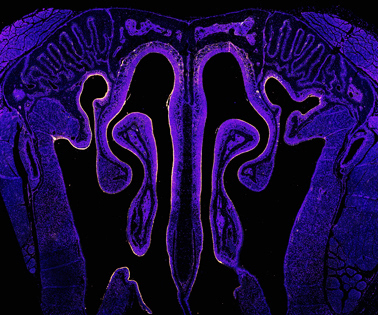 Evading clearance and degradation by mucus, the vaccine (yellow) is taken up by epithelial cells (indigo) lining the nose. It uses a naturally-occurring protein called albumin as a chaperone to hitchhike across mucosal cell layers (magenta) into underlying immune tissues. Further testing shows that the vaccine elicits protective immune responses against intruders such as HIV and SARS-CoV-2.
Evading clearance and degradation by mucus, the vaccine (yellow) is taken up by epithelial cells (indigo) lining the nose. It uses a naturally-occurring protein called albumin as a chaperone to hitchhike across mucosal cell layers (magenta) into underlying immune tissues. Further testing shows that the vaccine elicits protective immune responses against intruders such as HIV and SARS-CoV-2.
Credit: Brittany Hartwell, Jason Y.H. Chang, Darrell J. Irvine. Courtesy of the Koch Institute for Integrative Cancer Research at MIT.
Take SARS-CoV-2 for example. Researchers are pursuing dozens of mucosally delivered vaccine candidates, many designed to be given intranasally, against the virus in the hopes that they might be better able to protect against infection than those administered subcutaneously, but it isn’t easy to get vaccine immunogens across the mucosal barrier, where they can take up residency in tissues and stimulate a mucosal immune response.
Live-attenuated virus vaccines may be the best choice for mucosal vaccination, at least in terms of stimulating immune responses that can protect against infection altogether and not just disease. However, these vaccines may be inappropriate for use in large swaths of individuals, including infants, the elderly, and those who are immunocompromised.
It is thought that other vaccine approaches, including protein subunit vaccines, may require adjuvants — substances added to a vaccine to boost the resulting immune response — if delivered mucosally. But these too come with safety concerns, and there aren’t many adjuvants designed specially to navigate through mucosal barriers. Authors of a recent review article in Vaccine on the promises and challenges of mucosal SARS-CoV-2 vaccines wrote: “There are limited mucosal adjuvants that have a sufficient safety profile for development as human vaccines, and many of the most promising ones require further testing to demonstrate their safety and efficacy in humans.”
This is why Brittany Hartwell and her lab at the University of Minnesota are looking for an alternative.
“The challenge for the field has been delivering vaccine antigens and adjuvants across mucosal barriers,” says Hartwell, an assistant professor in the department of biomedical engineering. “We need to engineer vaccine molecules that can get across the actual mucus layer and avoid both clearance and degradation in that layer, and then also get them across the tight epithelial cell layer and the tight junctions between those epithelial cells into the underlying submucosal layer. And while virus-based vaccines can do that because they have evolved to get across those barriers, most protein subunit vaccines really can’t,” she says.
“A lot of studies show that within about 24 hours after delivering a protein-based antigen intranasally it gets cleared or degraded within the mucus, so there is a clear engineering component of just improving the mass transport of these vaccines.”
Hartwell’s lab is exploring whether attaching vaccine proteins to albumin, a blood protein that can successfully transit across mucosal tissues, might be the answer. By attaching the proteins to albumin, it allows them to hitchhike their way across the mucosal barrier.
“While mucosal membranes are very good at keeping most components out, there are two proteins that are very good at getting in, as they are commonly recycled across mucosal membranes by a neonatal Fc receptor known as FcRn. These two endogenous proteins are albumin and immunoglobulin G (IgG).”
This neonatal Fc receptor (FcRn) is what allows for the transfer of humoral immunity from mother to newborns in humans. It is also expressed in various tissues in adults. And albumin, in vivo, acts as a fatty acid transporter, meaning it is well suited for navigating through these tightly bound tissues. “We were able to engineer our vaccine platform so that is has an amphiphilic tail that binds on to the lipid binding sites of albumin and then uses albumin to hitchhike across the mucosal tissues using the FcRn,” says Hartwell.
In early studies, this seems to help improve the efficiency of getting vaccine antigens to where they need to be. “One of the things we showed in our recent paper is that by doing this we can enhance uptake of our vaccine platform across mucosal barriers.”
Once the vaccine components get across the barrier, the real work begins. “The vaccine antigens concentrate in the mucosal-associated lymphoid tissue (analogous to a lymph node, but for the mucosa), where they can enhance immune responses in germinal centers,” she explains. “And by enhancing germinal center responses in secondary lymphoid tissue it can then improve antibody responses long term.”
For respiratory infections, such as SARS-CoV-2, setting up long-lasting antibody responses directly at the mucosal surfaces in the nose should enable the immune system to respond much faster to the invading virus. “In this case, it primes specific mucosal antibodies that we can think of as a frontline defense,” she says, “in addition to back-up antibodies in the blood.” Hopefully even fast enough to be able to block infection or snuff it out before it has a chance to spread further in the respiratory tract.
But the benefits of mucosal vaccination don’t stop there. “I think one of the really cool things about the mucosal immune system is this concept of common mucosal immunity, where our mucosal system is connected via homing mechanisms, so if you activate or induce an antigen-specific response at one mucosal inductive site, then that triggers activation of local immune cells there to express homing markers that will then send them to more distal mucosal sites or mucosal tissues,” Hartwell elaborates.
In studies in mice, Hartwell and colleagues found that intranasal administration of the SARS-CoV-2 receptor binding domain (RBD) protein with the engineered amphiphilic tail could elicit 100- to 1,000-fold higher antigen-specific IgG and IgA responses in serum, as well as in the mucosa of the upper and lower respiratory tract. This approach also induced much higher antibody titers in the genitourinary tract of mice as compared to an unmodified RBD protein.
“The nose is considered an inductive site and activation or exposure to antigen in the nose triggers homing of mucosal immune cells, such as B or T cells or plasma cells, to the upper and lower respiratory tract, as well as to the more distal genitourinary tract. That is why we are able to use this common inductive site with an intranasal vaccine to elicit immunity for something like SARS-CoV-2 and even HIV, which is typically transmitted via the genitourinary tract.”
Inducing mucosal immunity will likely be essential for an effective HIV vaccine. Hartwell says her lab is currently investigating whether other routes of administration, including oral delivery of this albumin-based vaccine platform, might be more well suited to HIV vaccines that need to primarily induce immune responses in the genital mucosal tissues, or the tissues of the gastrointestinal system, which are one of the early sites of HIV replication and destruction of the critical cells of the immune system.
They are also exploring what types of vaccine antigens it may be feasible to deliver using this approach. For SARS-CoV-2, the receptor binding domain protein is an easy candidate because this relatively small protein is the primary target of neutralizing antibodies. Whereas for HIV, it isn’t as straightforward. “For HIV, it is thought that you need to deliver larger, more native-like trimer proteins,” Hartwell says.
So far, Hartwell and her team have used the albumin platform to deliver the monomeric form of the engineered outer domain of HIV gp120 protein, known as eOD, which was developed by Bill Schief, a scientist at Scripps Research, IAVI’s Neutralizing Antibody Center, and Moderna. The antigen, which is being tested in clinical trials as a 60mer nanoparticle, is considered a vital first step to train the human immune system to produce the types of broadly neutralizing antibodies against HIV that are induced only very rarely in response to chronic HIV infection.
They also have done some preliminary studies to conjugate a larger native-like HIV trimer protein onto their vaccine delivery platform. “We see enhancement of serum IgG and vaginal IgA responses with this delivery,” says Hartwell. “That’s just preliminary, unpublished data but I think it speaks to the potential for using this approach with larger protein conjugates.”
Hartwell’s interest in working on HIV vaccines was seeded during her postdoctoral work in the laboratory of Darrell Irvine at the Massachusetts Institute of Technology, where much of the work on using albumin to transit vaccine antigens across the mucosa was done. Now she is continuing this work in her own lab, extending its application to many pathogens, including influenza, respiratory syncytial virus, and cytomegalovirus, as well as SARS-CoV-2 and HIV. Given the necessity of inducing mucosal immune responses for optimal immunity against these pathogens, it is certain that Hartwell will be quite busy with mucosal hitchhikers.