November 21, 2024
Drug-resistant microbes are on the march. Vaccines to the rescue?
Vaccines against deadly microbial pathogens could put a dent in antimicrobial resistance.
By Michael Dumiak
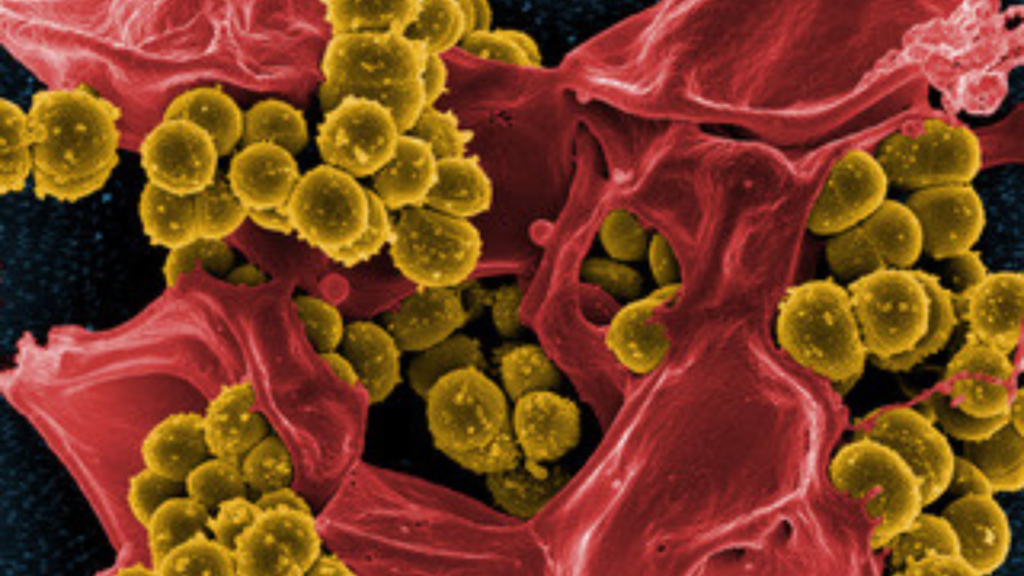
The researchers perhaps furthest along in developing a vaccine against Staphylococcus aureus bacteria work in a small biotech on the outskirts of Zürich. “A 50-person company, here in little Switzerland,” says Michael Kowarik, chief scientific officer and a co-founder of LimmaTech Biologics. “It tells you that the pipeline is not very dense.”
The pipeline isn’t just thin for vaccines to combat S. aureus, a common bug frequently responsible for healthcare-associated and surgical site infections of the bloodstream, as well as skin and soft tissues. It’s thin for preventive vaccines against all bacterial pathogens, according to the World Health Organization (WHO). That includes other prominent bacterial pathogens, including Mycobacterium tuberculosis, the bacteria that causes TB; Klebsiella pneumoniae; and Neisseria gonorrhoeae.
The pipeline of vaccines for bacteria is sparse partly because they are challenging targets. The economics are also unfavorable. Ideally, low-cost oral antibiotics are only taken when an infection occurs. However, vaccines, even if they are eventually economical to produce, must be delivered, distributed, and used widely to prevent infection altogether. But Kowarik and his colleagues still think vaccines will play a vital role in combatting an increasingly virulent and worrisome threat to global public health: antimicrobial resistance.
“We see more and more in the antimicrobial resistance field that there is a space for vaccines,” Kowarik says. “Only a couple of years ago it was all based on antibiotics.”
Concerned researchers and public health officials are looking to bolster efforts against antimicrobial resistance in any way possible and to prioritize it within the global public health agenda, as outlined in a special series in The Lancet in June. At a September United Nations high-level meeting — now the second on the theme — there were pledges to cut antibiotic and antimicrobial misuse and to boost funding to support national-level plans to combat antimicrobial resistance. Health ministers gathered in mid-November in Jeddah, Saudi Arabia, to follow up.
Antimicrobial resistance is emerging as a major killer, especially in poorer populations. A 2022 modelling study in The Lancet estimates that five million deaths globally are associated with drug-resistant bacteria.
At a panel at the recent World Health Summit in Berlin, Tamas Andras Koncz, a vice president of medical affairs at Pfizer, said it’s becoming one of the biggest healthcare burdens in sub-Saharan Africa, heading toward superseding HIV, malaria, and tuberculosis. Antimicrobial resistance took up an entire afternoon at the summit, with panels of experts, including the new head of Wellcome, John-Arne Røttingen; Khumbize Kandodo Chiponda, Malawi’s health minister; and Catharina Boehme, an assistant director-general of the WHO, among others. Wellcome is an investor in the AMR Action Fund, a US$1 billion industry seed foundation for therapeutics developers with the goal of launching two to four new antimicrobials by 2030.
Writing in the journal Vaccine earlier this year, WHO’s Mateusz Hasso-Agopsowicz, an immunologist and technical officer, joined colleagues at the Gates Foundation and universities in London and Brussels in arguing that vaccines are and will be crucial — and often underappreciated — in curbing the development of antimicrobial resistance.
Agopsowicz and colleagues have produced in-depth research over the last four years outlining the field of bacterial vaccines in clinical and preclinical development, as well as gauging the potential impact of these vaccines in reducing the development of antimicrobial resistance by decreasing the reliance on antibiotics. They have identified the most concerning pathogens — bacteria such as Acinetobacter baumannii, Excherichia coli, K. pneumoniae, Pseudomonas aeruginosa, and S. aureus — and outlined the paucity of vaccines or even vaccine candidates in clinical development for them. The report models how ideal vaccines could, for example, reduce antibiotic use by 142 million daily doses and save $860 million in hospital costs associated with antimicrobial resistance.
But producing these ideal vaccines, or even vaccines that are just better than current treatments, requires overcoming steep challenges. As Anke Osterloh of the Leibniz Association’s Lung Research Center points out, live bacteria are more complex organisms than viruses (if not as mutable) and have a variety of antigens whose immunogenic potential is unknown. While vaccines against extracellular bacteria have been developed successfully and are in wide use — the tetanus and diphtheria vaccines, for instance — creating vaccines against many of the pathogens most concerning for antimicrobial resistance will be more complicated.
“They have different virulence mechanisms that attack different features of the host and at different stages of infection,” Kowarik says. “Future vaccines here must be composed of many antigens, in my opinion. It must be a multi-antigen vaccine that addresses different virulence mechanisms at the same time. It should also cover multiple serotypes.”
To this end, LimmaTech has further developed what it calls self-adjuvanting and multi-antigen vaccine platforms to produce vaccine candidates. One of the company’s strategies is to pursue vaccines that can neutralize or inhibit the toxic activity created by a bacterial pathogen when it enters the body. Whereas many vaccines seek to produce antibodies that recognize and respond to surface molecules on the pathogen, LimmaTech’s candidate against S. aureus, which will enter Phase 1 studies in early 2025, aims to generate an immune response that can instead neutralize the toxins secreted in an infection.
To bolster its chances against the economic odds facing any small biotech, LimmaTech has long-established research and development partnerships with large pharmaceutical manufacturers GSK and Johnson & Johnson, as well as fostering more recent ventures alongside specialty vaccine producers such as the French biotech Valneva. “We look to develop the Phase 1 and Phase 2 vaccine candidates from the research lab,” says Patricia Martin Killias, a biochemist and LimmaTech’s chief operating officer, “and bring in a bigger partner to move to Phase 3.”
If successful, this vaccine will play an important role in combatting antimicrobial resistance. A successful vaccine against tuberculosis could also have a major impact on curbing antimicrobial-resistance-related deaths. MTBVAC, a live-attenuated M. tuberculosis vaccine, is currently in clinical trials. But improving basic infrastructure needs, such as clean water, sanitation, and access to care, alongside better animal husbandry, is also critical, the Malawian health minister Khumbize Kandodo Chiponda said in Berlin.
New antibiotics are also essential. Much like vaccines, these new medicines are developing slowly, and producing them is going to be more complex than in the past. Kevin Outterson is executive director of CARB-X, a global nonprofit partnership supported by several national governments alongside Wellcome, the Gates Foundation, and the Novo Nordisk Foundation. He worries about researchers leaving antibiotic research and the field itself becoming even more thinly resourced in years to come.
Speaking about novel antibiotics targeting the “worst” pathogens on WHO’s list, Outterson counts 20 projects in clinical development. “For cancer, there are several thousand, depending on where you look. But many multiples higher,” he said. “Right now, we have a crisis in human capital.” With antibiotics, there is also the conundrum of trying to develop new ones while there is a concerted push to discourage their use, which limits commercial incentives.
So, every step ahead will count.
LimmaTech’s Killias spent the last weeks organizing logistics for a Phase 2b trial of LimmaTech’s vaccine against Shigella, a major cause of moderate to severe diarrhea, including dysentery. There are four primary species of the bacteria, and it can be deadly: in 2019 some 29,000 fatalities were associated with antimicrobial-resistant Shigella infection.
In October, the U.S. Food and Drug Administration decided to fast-track the vaccine candidate. Researchers have just injected the first volunteer in what will be a challenge trial to show protection in a controlled human infection model. The vaccine is intended to protect travelers to regions where Shigella is endemic, which includes most of the tropical and subtropical areas of the globe. Fewer globe-trotting travelers gobbling antibiotics would be one step in outflanking resistance. Another trial testing LimmaTech’s Shigella candidate in infants gets underway next year in Nairobi. With 80-160 million cases of the disease annually — most occurring in children — that would be a potentially much larger step.
Read more: