March 14, 2024
Don’t Stop Believin’: This year’s HIV vaccine research theme
Researchers at CROI discuss progress on the long road to developing an HIV vaccine.
Kristen Kresge Abboud

The titles of HIV vaccine sessions at scientific conferences tend to epitomize the current sentiments of the field. Over the years these session titles have on occasion conveyed optimism, while at other junctures communicated a more dire call for a “back to basics” approach.
But most often, following a series of disappointing results from clinical trials, these session titles described a field at a crossroads. That is no longer the case. Researchers in the HIV vaccine field have largely coalesced around what they think will be required to achieve a preventive vaccine. Now, innovation and perseverance are providing reason for hope, which is likely why this year’s main vaccine session at the 24th annual Conference on Retroviruses and Opportunistic Infections (CROI) was entitled, The HIV Vaccine Journey: Don’t Stop Believin’.
Apart from the clever reference to an American rock band’s hit song, this session title accurately portrays today’s attitude toward HIV vaccine development. There is a flurry of activity and irrefutable progress in developing vaccine candidates specifically designed to induce broad and potent antibody responses against the virus. Simultaneously, researchers are pursuing novel strategies to induce HIV-specific antiviral cellular immune responses. Ideally, these two approaches will be combined to provide the best shot at blocking infection.
Advances in this work and novel approaches to vaccine delivery, all of which were featured at CROI, are indeed keeping the belief in HIV vaccine research very much alive.
Hitting the target
Steady progress in engineering HIV vaccine immunogens is giving rise to a surge in clinical evaluation. In a CROI plenary on HIV vaccines, Julie McElrath, senior vice president and director of the Vaccine and Infectious Disease Division at the Fred Hutch Cancer Center, said there are more than 14 discovery medicine trials of broadly neutralizing antibody (bnAb) vaccine candidates either already underway or planned to start this year. These vaccine candidates are designed to induce bnAbs against multiple sites of vulnerability on the virus.
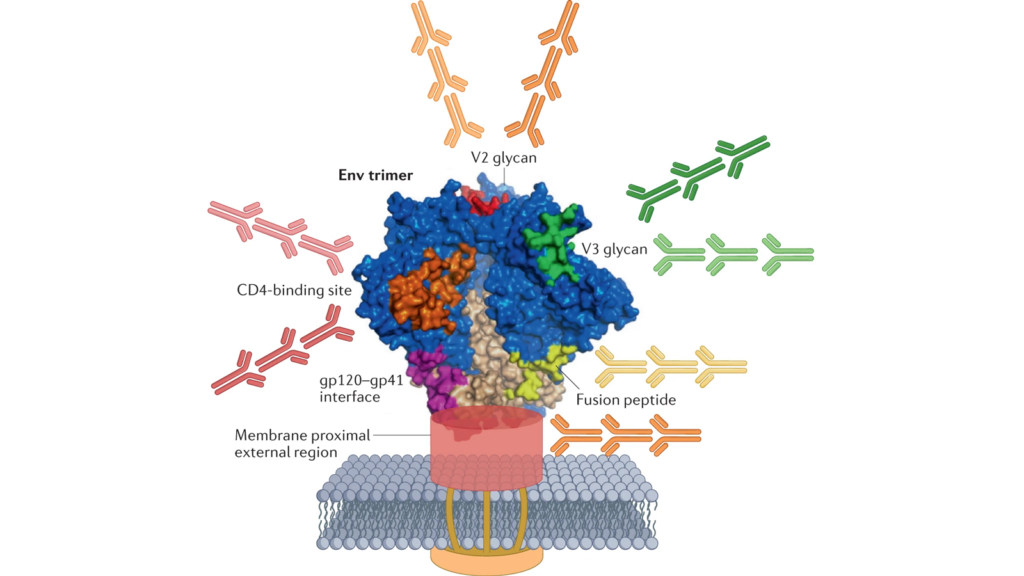
Several of these candidates are based on an approach known as germline targeting. This approach mimics the process of bnAb development that occurs rarely in natural infection — a small minority of people living with HIV develop bnAb responses after years of exposure to a continuously evolving virus.
Germline targeting is a multiple-step process. It starts with a vaccine immunogen capable of engaging the small subset of naïve B cells in our bodies that have the intrinsic capacity to become bnAb-producing B cells and then uses a series of antigens to usher these B cells through the process of maturation that is required to make this happen. Researchers describe this stepwise process in three stages: the priming stage, which involves engaging the naïve B cells; the shepherding or shaping stage, which is when these B cells are encouraged to undergo several mutations that mature and diversify the immune response; and the polishing stage, which involves administration of a more native-like HIV immunogen that ultimately results in the formation of B cells capable of eliciting bnAbs.
There are three leading germline targeting approaches currently under investigation that aim to induce bnAbs that target the CD4 binding site on the virus, and each was discussed in detail at CROI. One of them starts with the eOD-GT8-60mer nanoparticle antigen developed by Bill Schief and colleagues at IAVI and Scripps Research. In a Phase 1 trial known as IAVI G001, this eOD immunogen successfully primed the immune response, inducing a high frequency of the targeted B cells in the vast majority of the 36 volunteers who received the vaccine candidate. This established an important clinical proof-of-concept for this strategy.
In two follow-up Phase 1 studies, IAVI G002 and IAVI G003, researchers tested this same eOD-GT8-60mer immunogen delivered via Moderna’s mRNA platform technology. In IAVI G002, there was also an additional engineered immunogen delivered as part of a sequential immunization strategy. This second immunogen, Core-g28-v260mer, also delivered via mRNA, was designed to guide the early maturation of the primed B cells toward bnAb development, the so-called shaping of the immune response. Results from IAVI G002 and G003 haven’t been published yet but Rogier Sanders, a professor at Amsterdam University Medical Center who was not involved in the studies, reported at CROI that the results were encouraging. A full set of results, including both the immunogenicity data and information about skin reactions among some vaccinees that were discussed recently in Science and a statement from IAVI, will be published soon.
Two other CD4 binding site germline targeting strategies are also moving ahead. Sanders, Marina Caskey, professor of clinical investigation at Rockefeller University, and colleagues at IAVI are testing another immunogen known as BG505 SOSIP.664.v4.1-GT1.1 in a series of Phase 1 trials. This immunogen was designed by Sanders and John Moore of Weill Medical College of Cornell University and is a modified germline-targeting version of the native-like trimer BG505 SOSIP.664 first stabilized in 2013. In the first trial, IAVI C101, this priming immunogen was tested with the AS01 adjuvant, and according to Sanders’ presentation at CROI, most participants mounted a CD4 binding-site directed antibody response after three escalating doses, suggesting the priming of the immune response was successful.
In a pair of follow-up studies known as IAVI C107 and IAVI C110, researchers are boosting participants from IAVI C101 who have already received the BG505 SOSIP.664.v4.1-GT1.1 immunizations with two doses of the BG505 SOSIP.664 gp140 vaccine candidate administered with two adjuvants: 3M-052 AF and Alum. This boosting immunogen is designed to mimic the native trimeric structure of HIV’s Envelope protein and is therefore more of a finishing immunogen, but Sanders said he is hopeful that these studies will help researchers identify an optimal shaping immunogen for this regimen.
In another ongoing Phase 1 trial, known as HVTN301, the U.S. National Institute of Allergy and Infectious Diseases (NIAID) and the HIV Vaccine Trials Network are testing the germline targeting priming immunogen 426c.Mod.Core-C4b, a self-assembling nanoparticle developed by Leo Stamatatos and colleagues at the Fred Hutch Cancer Center, is also delivered with the 3M-052 and Alum adjuvants. This immunogen is being delivered as either a single, bolus injection or in fractionated doses (six immunizations of escalating doses delivered over 17 days).
Analysis of this trial is ongoing but both McElrath and Sanders said that the immunogen is inducing the desired precursor antibodies. McElrath also reported that the fractionated/escalating dose delivery method seems to induce better responses overall than the bolus delivery. A heterologous boosting study for this approach is expected to begin soon.
Other germline-targeting immunogens that aim to induce bnAbs to other sites on the HIV Envelope trimer are also now in clinical trials, including one targeting the V3 glycan.
Novel immunization strategies
As these trials progress, researchers are eyeing several ways, in addition to the design of the immunogen, to improve the process of B-cell maturation and proliferation in germinal centers which is key to improving the likelihood of success with these approaches.
At CROI, Darrell Irvine, a professor of biomedical engineering at the Massachusetts Institute of Technology (MIT), emphasized the importance of employing vaccination strategies that can help these less common, lower affinity B cells enter germinal centers, where they can mature and expand. He called this a “really challenging immunology problem,” but one for which researchers are actively seeking solutions.
A few of these potential solutions are already being explored in clinical trials, including testing novel adjuvants such as the saponin/MPLA nanoparticles (SMNP) or formulating antigens as nanoparticles, such as the eOD immunogen. Nanoparticles present many copies of antigen and trigger innate immunity pathways that allow more antigen to reach lymph nodes.
Another approach in testing is using extended/escalating dosing of immunogens. Irvine said that administering the same total dose of antigen and adjuvant over an extended time frame in gradually escalating doses dramatically improves the formation of long-lived germinal centers as compared to a single dose, citing a study in non-human primates from Shane Crotty and colleagues at the La Jolla Institute for Immunology, Scripps Research, and IAVI’s Neutralizing Antibody Center. HVTN 301 is one of the ongoing clinical trials employing this strategy.
Irvine also described an effort to achieve similar results with a single shot,. Professors Robert Garcea and Theodore Randolph at the University of Colorado, Boulder, have developed a way of spray-drying antigen/adjuvant formulations to form glassy microparticles. These microparticles then get coated with multiple layers of Alumina, which are designed to slowly break down, modulating a gradual release of antigen and adjuvant. This strategy achieves the same effect as multiple doses and is also thermostable, eliminating the need for cold storage.
Garcea, Randolph, and colleagues are already testing this approach with the HPV vaccine and Irvine said this technology could potentially make complex vaccination strategies such as those that will likely be required for HIV much more practical.
Antiviral T-cell activity
Antibody-based vaccines may dominate the HIV vaccine field today, but researchers are also pursuing several approaches to stimulate cellular immunity. McElrath focused on two of these in her plenary.
The first is the live-attenuated cytomegalovirus vector vaccines. These vaccine candidates, as detailed previously in IAVI Report, seem to work through an unusual pathway and after decades of preclinical research are now being tested in Phase 1 clinical trials in humans.
The other approach McElrath highlighted is a T-cell immunogen developed by Gaurav Gaiha and colleagues at the Ragon Institute of Mass General, MIT, and Harvard. This immunogen is designed based on the key epitopes of HIV targeted by CD8+ T cells in individuals who spontaneously control HIV infection in the absence of antiretroviral therapy. These so-called highly networked epitopes are then encoded in a gorilla adenovirus vector (GRAd-HIV) developed by the Italian company ReiThera. This candidate will be tested in a Phase 1 human clinical trial in partnership with IAVI, with funding from the Bill & Melinda Gates Foundation.
Persistent challenges
Amidst all this progress, there are still many challenges ahead. At the close of her talk, McElrath noted some of them, including accelerating the production of safe vaccine designs for clinical testing, selecting intermediate and final vaccine immunogens to induce mature HIV-specific broadly neutralizing antibodies in serum, identifying optimal combinations of vaccine approaches to induce both bnAb and HIV-specific T-cell responses, and maintaining surveillance to ensure that immunogen design efforts keep pace with viral evolution.
Finally, McElrath noted one more challenge that signals the long road ahead perhaps even more clearly than all the others — the need to invigorate young investigators to take on the HIV vaccine challenge and ensure funding is secured to achieve success in this effort. In other words, the message to funders and next-generation scientists: Don’t stop believing.