IAVI’s epidemiology studies inform target selection and prioritization for product development

Our epidemiologists study the natural history, distribution, determinants of disease, and the impact of interventions on disease burden and health.
The epidemiology team works with the product development, clinical development, and global access teams to identify where epidemiological research is needed to fill information gaps and de-risk end-to-end programs. We also partner with clinical research centers, global health organizations, and academic institutions. Together, we co-develop, design, and prioritize research studies.
In addition to observational studies, we carry out systematic reviews and analyses of existing scientific literature. These activities help us to understand the body of knowledge around a public health problem. We also employ mathematical modeling to predict how a proposed health intervention, such as a vaccine or antibody, may work or where we should run a particular clinical trial.
Our mission
The mission of the epidemiology team is to support the IAVI product pipeline by bridging key epidemiological knowledge gaps for discovery, early and late-stage development, and target selection. This will be achieved by:
- Addressing and bridging epidemiological knowledge gaps to enhance product development.
- Conceptualizing and designing innovative epidemiological activities to obtain robust data for informed decision-making.
- Providing valuable insights into epidemics, outbreaks, and contributing to a deeper understanding of the natural history.
- Initiating partnerships and consortia for epidemiological activities ensuring a footprint for further collaboration.
- Establishing a center for excellence for epidemiological and statistical methodologies to support product development strategies.
Our accomplishments
Clinical course of HIV infection
(Protocol C)
The largest cohort of adult Africans with over 600 participants with early incident HIV infection, over 400 of whom enrolled with their suspected transmitting partner. This study characterized disease course, immunology, and HIV-neutralizing antibodies in people with well-described dates of HIV acquisition.
Neutralizing antibodies for HIV
(Protocol G)
A survey of over 2,100 people living with HIV in 12 sites across the globe to characterize the prevalence and characteristics of HIV broadly neutralizing antibodies.
Safety reference intervals
(Protocol D)
The first large, multi-site study to establish laboratory reference intervals (RIs) in healthy adult Africans eligible for clinical trial participation. Region-specific RIs are important for clinical trials and these data are often sparse in priority areas for research, including Africa.
Lassa fever epidemiology
(X100 and X102)
The largest study of Lassa fever virus incidence in Sierra Leone conducted in harmony with the CEPI-funded ENABLE cohort study.
Engaging adolescent girls and young women in clinical research (X101)
The collaborative Multisite study with Adolescent Girls and Young Women (MAGY) will enroll and follow participants from this priority population for 24 months, and will measure outcomes including STI, pregnancy, HIV, and interest in future participation in clinical research for HIV prevention vaccine and antibody candidates in sub-Saharan Africa.
Assessing epidemiology for
large-scale TB vaccine clinical trials
Assessing regional epidemiology to inform CRC selection for the IAVI C113 Phase 2B trial of the TB vaccine candidate MTBVAC. This work is vital to fulfilling vaccine trial efficacy endpoints, ensuring a judicious sample size, and maintaining a feasible follow-up duration. One of the primary objectives is to de-risk the trial by mitigating the possibility of insufficient endpoints.
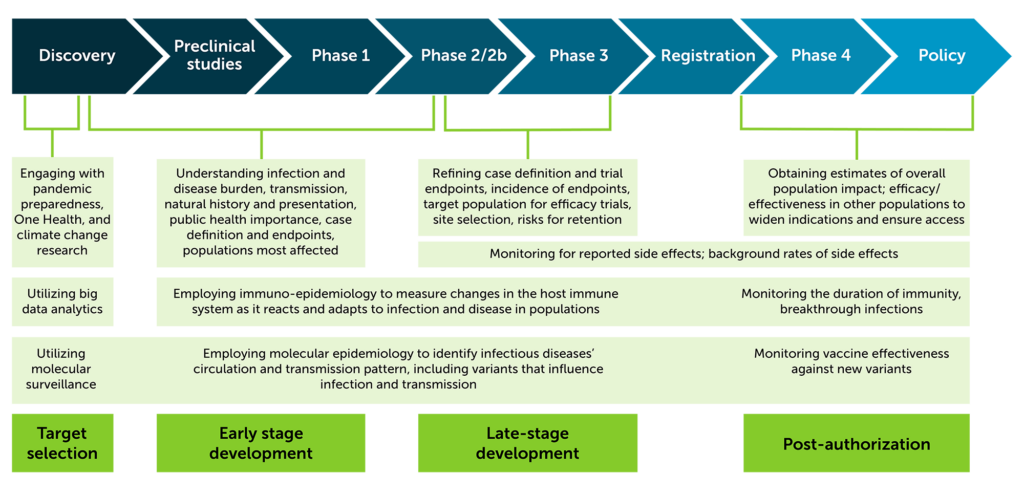
How does epidemiology inform the product development process?
At IAVI, epidemiology activities support product development at all stages and is an important piece of the integrated product development plan. Below, the figure shows epidemiology activities for target selection, early and late stage development, and post-authorization. Expand the section below for a graphic illustration of the product pipeline.
Our studies
IAVI’s epidemiological research was supported by USAID beginning in 2000 and has resulted in the completion of over 60 observational epidemiology studies in eight countries in sub-Saharan Africa and India. Studies are listed alphabetically. Expand each entry to view full details.
Purpose: To estimate HIV prevalence, prepare research teams for high-volume HIV counseling and testing for large scale clinical research, and evaluate and educate communities for efficacy trial participation.
Type: Prevalence/cross-sectional study. 6,436 participants enrolled to estimate HIV prevalence at four sites.
Study Status: Complete
Partners: KAVI-Institute of Clinical Research, Kangemi, Nairobi, Kenya; Kenya Medical Research Institute-Wellcome Trust Research Programme, Kilifi, Kenya; Medical Research Council, Masaka, Uganda; Joint Clinical Research Center, Kakira, Uganda
Purpose: To estimate the annual incidence of HIV infection, characterize early HIV infection, and prepare clinical trial sites for HIV preventive vaccine efficacy trials for which participants from this study cohort may be recruited.
Summary: IAVI and our partners have long recognized the importance of understanding the local epidemiology of HIV to best inform future HIV prevention trials. To this end, we have partnered with many teams since 2004 to study HIV incidence, associated predictors of HIV acquisition, participant retention, and the needs and motivations of key and priority populations as they relate to participating in biomedical research and improved access to health care.
Type: Incidence/prospective cohorts in higher-risk populations potentially suitable for efficacy trial participation. This family of studies includes nearly three dozen cohorts that have enrolled over 26,000 participants including female sex workers, men who have sex with men, at-risk women, married couples, and fishing community members.
Study Status: Multiple studies completed, all are now closed.
Partners: HIV Pathogenesis Program, Durban, South Africa; Kenya Medical Research Institute-Wellcome Trust Research Programme, Kilifi, Kenya; MRC, UVRI & LSHTM (MUL) Uganda Research Unit, Masaka, Uganda; Center for Family Health Research in Zambia, Lusaka and Ndola; Uganda Virus Research Institute-IAVI, Entebbe, Uganda; KAVI-Institute of Clinical Research, Kangemi, Nairobi, Kenya; Medical Research Council, Entebbe, Uganda; Desmond Tutu HIV Centre, Cape Town, South Africa; Aurum Institute, Rustenburg, South Africa; Center for Family Health Research, Kigali, Rwanda
Purpose: To evaluate clinical, laboratory, immunologic, and viral markers of disease progression in participants with recent HIV infection to prepare for activities relevant to the execution of preventive HIV vaccine efficacy trials. If identifiable and willing, HIV-infected partner(s) of enrolled participants were assessed for virologic and immunogenetic parameters relevant to transmission.
Summary & Impact: Epidemiological studies of people who are in the early and acute stages of HIV infection are a high-value resource for vaccine design and development, as well as for cure research. In the Protocol C project launched in 2006, IAVI and a wide range of research partners studied participants in Kenya, Uganda, Rwanda, Zambia, and South Africa to learn more about how HIV is transmitted and HIV disease progression during a period of nascent ART programs on the continent. Enrollment closed in 2011 with more than 600 participants with incident HIV infection. All participants were provided with or referred for routine HIV care, including ART provision. Some 190,000 Protocol C samples were collected and more than 26,000 shared with researchers around the world, and follow-up ended in 2020.
Type: Prospective cohort of participants with incident HIV infection. 613 participants enrolled from nine research centers for long-term follow up; 406 suspected transmitting partners for a single visit.
Study Status: Complete
Partners: KAVI-Institute of Clinical Research, Kangemi and Kenyatta National Hospital, Nairobi, Kenya; Kenya Medical Research Institute-Wellcome Trust Research Programme, Kilifi, Kenya; Medical Research Council, Entebbe and Masaka, Uganda; Uganda Virus Research Institute-IAVI, Entebbe, Uganda; Projet San Francisco Center for Family Health Research, Kigali, Rwanda; Center for Family Health Research in Zambia, Lusaka and Ndola; Aurum Institute, Rustenburg, South Africa; Desmond Tutu HIV Centre, Cape Town, South Africa
Purpose: To establish clinical laboratory reference ranges among adults to determine relevant inclusion and exclusion criteria and to interpret safety data for HIV vaccine trials in the context of medical conditions present in an asymptomatic adult population at multiple sites in Africa.
Summary & Impact: Cross-sectional study to characterize laboratory reference intervals in healthy African adults, with a single follow-up visit to assess the effect of season in selected sites. 2,105 enrolled with 903 participants in the seasonal study and 200 in another study to assess the effect of splenomegaly.
Type: Cross sectional study, with a subset followed at a second visit to compare how seasonality may affect safety reference intervals.
Study Status: Complete
Partners: KAVI-Institute of Clinical Research, Kangemi, Nairobi, Kenya; KAVI-Institute of Clinical Research, Kenyatta National Hospital, Nairobi, Kenya; Kenya Medical Research Institute-Wellcome Trust Research Programme, Kilifi, Kenya; Uganda Virus Research Institute-IAVI, Entebbe, Uganda; Medical Research Council/Uganda Virus Research Institute and London School of Hygiene & Tropical Medicine Uganda Research Unit, Uganda; Center for Family Health Research, Kigali, Rwanda; Center for Family Health Research in Zambia, Lusaka
Purpose: To screen for broadly neutralizing monoclonal antibodies (mAbs) from healthy, adult participants who are HIV infected.
Summary & Impact: In 2006, IAVI and its Neutralizing Antibody Consortium launched the Protocol G project to search for broadly neutralizing antibodies (bnAbs) against HIV, partnering with clinical research centers in Africa, India, Thailand, Australia, the United Kingdom, and the United States. More than 2,100 healthy, HIV-positive participants from 12 research centers contributed blood samples to be screened. In 2009, scientists from IAVI, The Scripps Research Institute, and Theraclone Sciences collaborated to isolate and characterize the first new bnAbs to HIV seen in a decade and the first to be isolated from donors in developing countries, where the majority of new HIV infections occur. To date, more than 80 new bnAbs have been isolated and characterized from Protocol G specimens, and many have been shared with researchers across the HIV vaccine field, several of which have entered clinical trials.
Type: Cross-sectional. Single visit of ART-naive adults with HIV infection for 3+ years with no significant health issues. Those whose screened serum showed neutralizing activity were asked to come back for further testing and follow-up.
Study Status: Complete
Partners: Uganda Virus Research Institute-IAVI, Entebbe, Uganda; Center for Family Health Research in Zambia, Lusaka and Ndola; Projet San Francisco Center for Family Health Research, Kigali, Rwanda; KAVI-Institute of Clinical Research, Kenyatta National Hospital, Nairobi, Kenya; YRG Care, Chennai, India; Vaccine Trial Centre, Mahidol University, Bangkok, Thailand; Centre de Diagnostic et de Recherche sur le SIDA et les infections opportunistes, Abidjan, Côte d’Ivoire; Institute of Human Virology, Nigeria; National Serology Reference Laboratory, Australia; St. Stephen’s Centre, London, U.K.; SUNY Downstate Medical Center, Brooklyn, NY, U.S.; Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand
Purpose: Follow-up of clinical trial participants who acquire incident HIV infection.
Type: Prospective cohort of participants with incident HIV infection who had previously participated in HIV vaccine trials with the same schedule of procedures as IAVI’s early HIV infection cohort, Protocol C.
Study Status: In follow-up
Partners: Projet San Francisco/Center for Family Health Research, Kigali, Rwanda; KAVI-Institute of Clinical Research, Kangemi, Nairobi, Kenya; Center for Family Health Research in Zambia, Lusaka and Ndola; MRC, UVRI & LSHTM (MUL) Uganda Research Unit, Masaka, Uganda
Purpose: To detect and measure immune function in gastro-intestinal (GI) mucosal tissues in a convenience sample of patients undergoing endoscopic examination of the lower GI tract (e.g., colonoscopies, sigmoidoscopies) as part of their clinical care at Kenyatta National Hospital or Nairobi Hospital.
Type: An exploratory, cross-sectional study to detect and measure immune function in gastro-intestinal mucosal tissues in Kenyatta National Hospital endoscopy patients.
Study Status: Ongoing
Partners: KAVI-Institute of Clinical Research, Kenyatta National Hospital, Nairobi, Kenya
Purpose: To identify exposed seronegative participants, defined as individuals who remain HIV seronegative despite repeated exposure to HIV, and characterize the immunologic markers of exposure.
Type: Prospective cohort, 45 participants enrolled at two research centers in Kenya
Study Status: Complete
Partners: KAVI-Institute of Clinical Research, Kenyatta National Hospital, Nairobi, Kenya; Kenya Medical Research Institute-Wellcome Trust Research Programme, Kilifi, Kenya
Purpose: To collect samples for protocol for assay development and testing of sample collection methods.
Type: Typically cross-sectional. Participants enrolled to provide samples for assay development and immune response characterization to support future and ongoing clinical research.
Study Status: Over 20 small studies, typically between 20-40 participants, have been initiated, completed, or are in follow-up.
Partners: KAVI-Institute of Clinical Research, Kenyatta National Hospital, Nairobi, Kenya; KAVI-Institute of Clinical Research, Kangemi, Nairobi, Kenya; Medical Research Council, Entebbe, Uganda; Projet San Francisco Center for Family Health Research, Kigali, Rwanda; Center for Family Health Research in Zambia, Lusaka; Uganda Virus Research Institute-IAVI, Entebbe, Uganda; St Mary’s-London, U.K.
Purpose: To assess vaccine-induced mucosal HIV-1-specific immune responses in participants concurrently enrolled in HIV preventive vaccine trials.
Secondary Objectives:
To determine participants’ acceptance of mucosal sampling methods to help establish the reproducibility and validity of methods of assessing vaccine-induced mucosal HIV-1-specific immune responses.
To compare vaccine-induced systemic HIV-1-specific immune responses with HIV-1-specific immune responses at mucosal surfaces.
Type: Prospective mucosal sampling sub-study attached to IAVI-sponsored clinical trials, 89 participants enrolled
Study Status: Complete
Partners: KAVI-Institute of Clinical Research, Kangemi, Nairobi, Kenya; KAVI-Institute of Clinical Research, Kenyatta National Hospital, Nairobi, Kenya
Purpose: To identify acute (pre-seroconversion) HIV infection. Early identification is important because initiating early treatment has positive benefits for the volunteer, and early events in HIV infection may provide clues for developing vaccines, new therapeutics and cure research. New technologies have made identifying acute HIV infection increasingly easier and less expensive.
Summary & Impact: Following the closure of IAVI’s early infection cohort study, Protocol C, ongoing viral surveillance for assay and reagent development remained a priority for the field. Technology had begun to evolve such that the cost and logistics of identifying persons with very early HIV infection became more manageable. Within a year of ending Protocol C enrolment, IAVI partners began screening for acute (i.e., pre-seroconversion) HIV infection among adolescent girls and young women in South Africa. Since that time, this program has expanded to new sites in Kenya, Zambia, Rwanda, and Uganda. IAVI has entered into an agreement with Cepheid to provide GeneXpert real-time PCR platforms to allow rapid diagnosis of pre-seroconversion HIV infection. While treatment is now offered immediately and so-called “natural history” cohort studies are no longer ethical, identifying persons very soon after infection both serves to provide samples of currently circulating virus to study and to immediately put these people on treatment during a very infectious period when they typically go undiagnosed.
Type: Prospective cohort studies of participants with incident HIV infection identified before, or during, antibody seroconversion, using real-time PCR on the GeneXpert platform, or overnight traditional PCR. Participants identified during this acute phase of HIV infection are offered treatment and are followed over time.
Study Status: Complete
Partners: HIV Pathogenesis Program, Durban, South Africa; Kenya Medical Research Institute-Wellcome Trust Research Programme, Kilifi, Kenya; MRC, UVRI & LSHTM (MUL) Uganda Research Unit, Masaka, Uganda; Center for Family Health Research in Zambia, Lusaka and Ndola
Purpose: To use discarded surgical mucosal tissue to study immune responses relevant for HIV research.
Type: Convenience sample to obtain discarded surgical mucosal tissue from patients with and without HIV infection who are undergoing surgical procedures that involves the removal of such tissue
Study Status: Ongoing
Partners: KAVI-Institute of Clinical Research, Kenyatta National Hospital, Nairobi, Kenya
Purpose: To characterize Lassa fever virus epidemiology and incidence in Sierra Leone in harmony with the Coalition for Epidemic Preparedness Innovations (CEPI)-funded ENABLE epidemiology cohort study
Summary & Impact: Little is known about the epidemiology of Lassa fever virus (LASV). Several experimental vaccines are in development against Lassa fever. To improve our understanding of this disease, and to prepare for future vaccine clinical trials, X100 is a prospective cohort study that enrolls rural Sierra Leonians aged 2 and up in villages at risk of infection from Lassa fever virus. This study was designed to harmonize with and conducted alongside the ENABLE study, another prospective study of LASV epidemiology that enrolled participants from villages in other regions of Sierra Leone; co-enrollment in both studies was not allowed. In a related third project (IAVI X102), we are collaborating with the Kambia Research Center in the north of Sierra Leone to test stored samples from 1,991 participants of Ebola vaccine trials. This will provide additional data from a region not covered by X100 or Enable enrollment.
Type: Prospective cohort study in 8,225 rural residents of Sierra Leone. The ENABLE study is a prospective study with 5,003 participants, while the Kambia project is a retrospective cohort study with 1,991 participants, 708 of whom have up to four prospective visits with stored samples.
Study Status: X100 enrollment closed in 2022, follow-up completed in 2023, data is currently being analyzed. ENABLE is also closed; Kambia project is ongoing from stored samples.
Partners: X100 partners include Kenema Government Hospital, Kenema, Sierra Leone; Tulane University, New Orleans, U.S. Kambia project partners include London School of Hygiene and Tropical Medicine, U.K.; Kambia Clinical Research Center, Kambia, Sierra Leone; Sierra Leone College of Medicine and Health Sciences, Freetown, Sierra Leone
Purpose: To work across multiple sites with cohorts of at-risk adolescent girls and young women (AGYW) to characterize higher risk behavior, and to evaluate their interest in future vaccine and antibody HIV prevention research in sub-Saharan Africa.
Summary & Impact: In this multisite study, IAVI was to enroll up to 1,200 AGYW between the ages of 15-24 years in a prospective cohort study and follow them up for 24 months to evaluate future clinical trial participation. This was to include characterizing factors related to effective trial design to improve interest and retention in future HIV vaccine and antibody clinical trials and evaluation of risk for HIV, STI, and unintended pregnancy. A subset of participants would be invited to provide samples by fine-needle aspiration, large blood draws, and/or leukapheresis, to enable detailed characterization of immune status in adolescent girls and young women. In addition, select participants including adolescents and young women, parents, and opinion leaders were to participate in focus group discussions and in-depth interviews to better understand interest in clinical research participation. This study closed in 2025 before full enrollment due to the shutdown of USAID.
Type: Multisite study, 24-month prospective study with adolescent girls and young women
Study Status: Closed early
Partners: Center for Family Health Research in Zambia; Uganda Virus Research Institute-IAVI, Entebbe, Uganda; KAVI-Institute of Clinical Research, Nairobi, Kenya; Aurum Institute, Rustenburg, South Africa; Population Council; University of Manitoba; USAID
Purpose: To conduct long-term follow-up and assess the long-term health status of participants who have been enrolled previously in an IAVI-sponsored trial of an investigational HIV vaccine candidate.
This includes measuring clinical events (i.e., “adverse events” as per clinical trial procedures), incident HIV infection, the persistence of vaccine-induced antibodies, and to characterize immunological, virological, and immunogenetic responses.
Type: Clinical trial participant follow-up
Study Status: Ongoing
Partners: KAVI-Institute of Clinical Research, Kenyatta National Hospital, Nairobi, Kenya; Uganda Virus Research Institute-IAVI, Entebbe, Uganda; Center for Family Health Research in Zambia, Lusaka; Projet San Francisco Center for Family Health Research, Kigali, Rwanda; Desmond Tutu HIV Centre, Cape Town, South Africa; Medunsa, South Africa