IAVI-identified antibodies offer a new path to HIV prevention
IAVI and our partners have pioneered the discovery of many broadly neutralizing antibodies (bnAbs). This work has transformed the field of HIV vaccine research by helping scientists identify new targets on HIV. What’s more, the discovery of bnAbs has opened the door to a new possibility: the administration of HIV bnAbs to prevent HIV acquisition. We’re working to make that a reality.
At a glance
- 39.9 million people were living with HIV/AIDS in 2023
- 1.3 million people contracted HIV in 2023
- 200+ broadly neutralizing HIV antibodies discovered
- 3-6 months of protection likely with 1 dose of bnAbs
- $29 billion needed for global HIV/AIDS response by 2025
Antibodies are a proven way to prevent and treat some infectious diseases
Neutralizing antibodies are proteins that immune cells make to block viruses from infecting cells. The administration of neutralizing antibodies to prevent infection is known as passive immunization (see graphic, below). While a vaccine trains the immune system to generate a long-lasting, protective immune response, passive immunization delivers the antibodies directly into the body through infusions or injections. This protection fades over time, and so antibodies must be given periodically to maintain protection while a person is at risk.
Several antibody products for prevention and treatment of COVID-19 were authorized by the regulatory agencies for use during the pandemic. Antibodies are also used for the prevention of respiratory syncytial virus disease (RSV) in infants and young children and for treatment and prevention of rabies and tetanus, for example.

We have identified and isolated potent bnAbs
People living with HIV typically produce neutralizing antibodies to the virus. Unfortunately, HIV can rapidly mutate and become resistant to these neutralizing antibodies. After years of living with HIV, though, some people produce highly potent bnAbs that, in laboratory tests, are able to neutralize a wide variety of HIV strains.
Researchers at IAVI’s Neutralizing Antibody Center (NAC), a partnership with Scripps Research, and partners discovered a number of potent bnAbs by studying samples from large cohorts of individuals living with HIV in Africa, India, Thailand, Australia, the U.K., and the U.S.
We are developing bnAbs for prevention of HIV acquisition
HIV-specific bnAbs could have a role in treating, preventing, or even curing HIV. In 2021, a study showed that it is possible to protect people from acquiring HIV by giving them infusions of bnAbs. This worked only when circulating viruses and the bnAbs were very well matched, and the level of bnAbs in the blood was quite high. Still, it was proof of concept that bnAbs can block HIV in people.
IAVI has sponsored a number of clinical trials using our own antibodies as well as those of partners, with funding from the U.S. National Institutes of Health and the HIV Vaccine Trials Network. Learn more about IAVI’s clinical trials.
Now, technological advances have enabled researchers to optimize HIV bnAbs to make them more potent and longer lasting. IAVI is working toward proof of concept of an injection of a combination of engineered bnAbs that could provide protection from HIV acquisition for three to six months. IAVI and partners are planning a Phase 1 trial, Medio, of a combination of HIV bnAbs given to healthy adults in late 2025.
IAVI and our partners think that bnAbs are especially promising for the prevention of HIV acquisition in infants and breastfeeding children. Currently, large gaps exist in protecting newborns and children in areas where access to HIV prevention and treatment services is limited.
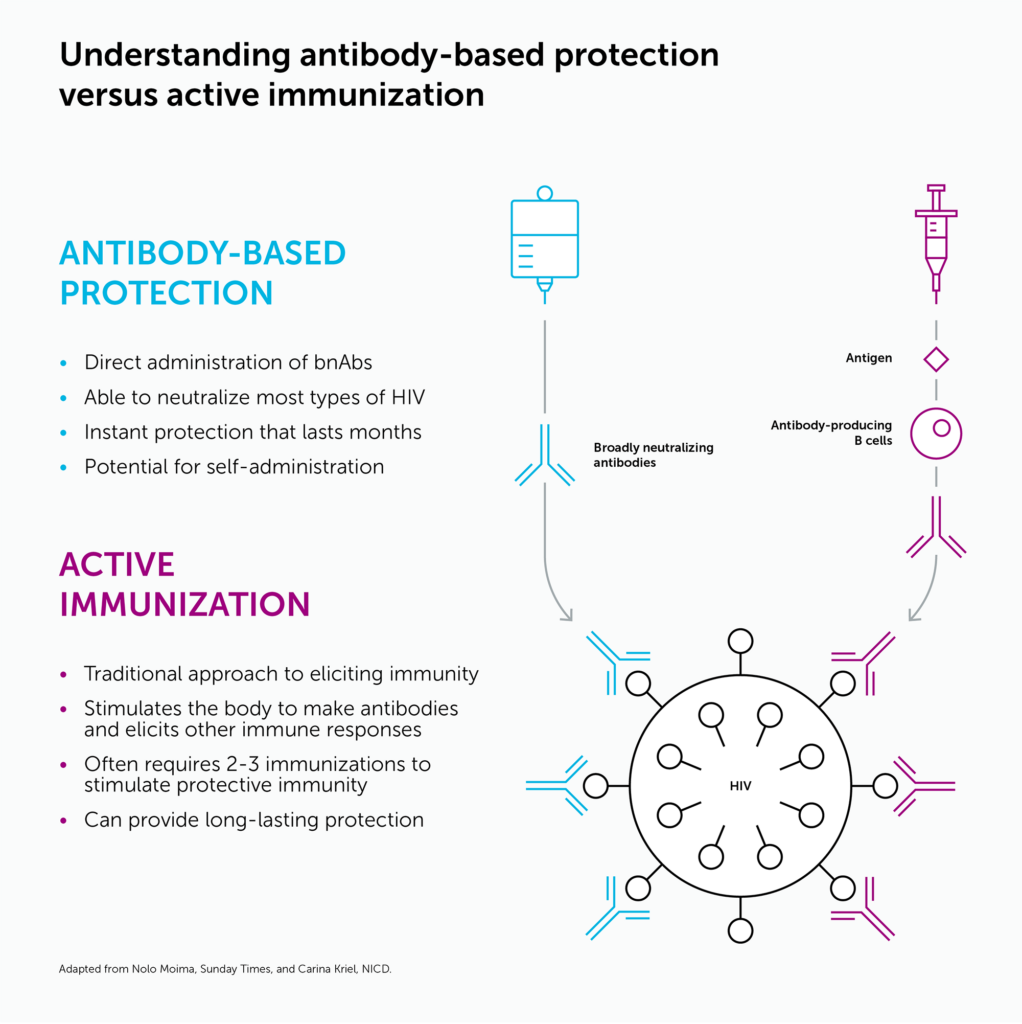
Access to antibodies
In complement with preclinical and clinical development of bnAbs, IAVI and our partners are working to ensure that bnAbs will be promptly, globally, and affordably accessible to the people who need them once they are shown to be efficacious.
Our commentary, Antibodies for HIV prevention: The path forward, published in the Journal of the International AIDS Society, highlights the lack of equitable access to antibodies in low- and middle-income countries and describes concrete actions that can help expand access to bnAbs once they are shown to be efficacious.